INTRODUCTION
Saphenofemoral ligation and stripping of the great saphenous vein (GSV) is considered to be the standard treatment for valvular insufficiency in the GSV [
12]. Although endovenous procedures, such as laser ablation and radiofrequency ablation, have been introduced and applied as new minimally invasive techniques for varicose veins, the highest success rate with the lowest recurrence rate can be accomplished with GSV stripping [
3]. Cryosurgery for varicose veins was introduced in 1978 and first clinically applied in 1982 [
4]. It works by the passage of nitrogen oxide (N
2O) through a very small nozzle under very high pressure, which decompresses and lowers the temperature of the probe to −85℃ in only a few seconds. During freezing, an ice ball forms at the tip of the probe, adhering the vein to the tip. Removal of the vein is obtained by withdrawing the probe in the direction of the entrance [
5]. This technique has the potential for good cosmetic results with less trauma, and lowers the rate of postoperative complications. However, it is not widely used, with few reports about cryosurgery for varicose veins. The aim of the current study was to present a single surgeon's experiences and outcomes of cryosurgery for the treatment of varicose vein.
Go to :

METHODS
This is a retrospective, noncomparative study designated to evaluate the efficacy and safety of cryosurgery for varicose veins. A total of 84 patients (131 limbs) with varicose veins with GSV incompetence were treated with cryosurgery by 1 vascular surgeon over a 2-year period (January 2012 to May 2014) using a cryomachine and a cryoprobe (Metrum CryoFlex; Spolka, Blizne, Poland) (
Fig. 1). Indications for the operation included saphenofemoral reflux in Doppler sonography with lower extremity pain, edema, heaviness, unsightly veins, and leg cramps at night. Patients classified as C1 class with typical symptoms of saphenofemoral reflux also underwent cryostripping of GSV prior to sclerotherapy. The exclusion criteria included any evidence of deep vein thrombosis (DVT) based on venous duplex ultrasound, current anticoagulation therapy, and excessive anesthesia risk. All patients underwent a routine preoperative clinical evaluation. Venous duplex ultrasound was performed to evaluate reflux and to rule out acute or chronic DVT. The following parameters were recorded and analyzed: the patient's age, gender, symptom, Clinical- Etiology-Anatomy-Pathophysiology (CEAP) classification, operative time, number of stab incisions, and postoperative adverse events like bruising, edema, cellulitis, seroma, superficial thrombophlebitis, cutaneous nerve damage, hematoma, and wound infection. The patients were followed for detection of postoperative complication and surveillance of recurrence following surgery at the outpatient clinic.
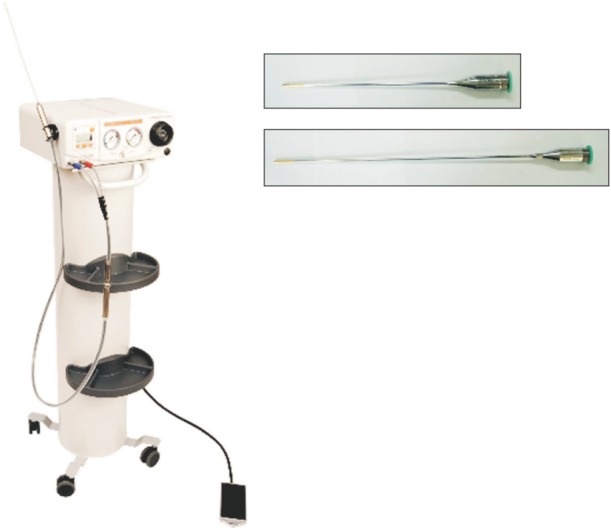 | Fig. 1The cryomachine and cryoprobe used for the cryosurgery of varicose vein (MetrumCryoFlex; Spolka, Blizne, Poland).
|
Surgical technique
All procedures were performed under spinal or general anesthesia. A 1- to 1.5-cm transverse skin incision was made at the level of the saphenous hiatus. After entrance to the saphenofemoral junction was confirmed, the proximal part of the GSV was ligated and tributaries of the saphenous vein were divided and ligated. After completing high ligation, a cryoprobe was inserted into the saphenous vein and passed down to the level of the knee (
Fig. 2). After the probe tip reached the desired segment of the GSV, tumescence (injection of 10 to 20 mL of normal saline) was induced around the cryoprobe to protect the surrounding structures. Freezing was initiated by the surgeon by pressing a footswitch. After maintaining the freezing cycle for 10–15 seconds, the saphenous vein was invaginated by pulling the probe upwards with a sudden move (
Fig. 3). Bleeding was controlled by immediate manual compression at the site of saphenous vein disconnection. Superficial varices were treated by extraluminal freezing and extraction with the cryoprobe through a few small stab incisions (3 mm) if needed. If too much skin was pulled in, freezing was stopped and the procedure restarted with the probe inserted slightly deeper and farther away from the skin. For accurate extraluminal freezing by cryoprobe, we used a transilluminator device that is composed of a 300-W xenon light and a pressure infusion system used in operations with Trivex (Smith & Nephew, Andover, MA, USA) (
Figs. 4,
5). After wound closure, a compression bandage was applied from the foot to the groin. Conservative management, such as wearing a compression stocking for 4 weeks, physical exercise or elevation of the affected limb, was recommended as supplementary treatments.
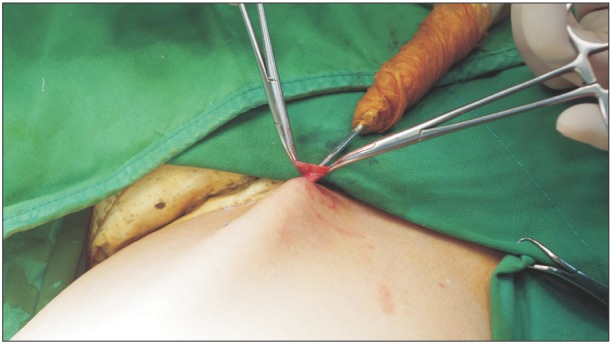 | Fig. 2A long cryoprobe is inserted into the lumen of greater saphenous vein with care to avoid vein perforation after all the collaterals are separated, ligated and cut off to prevent recurrence.
|
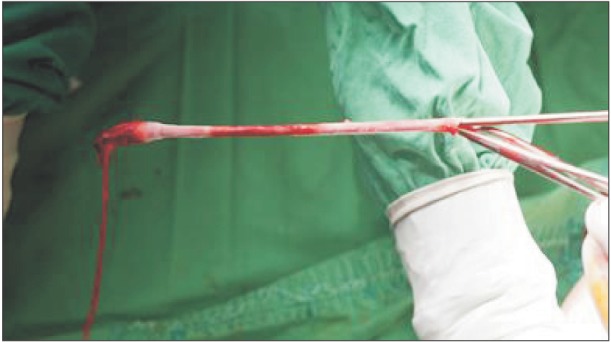 | Fig. 3Picture of removed greater saphenous vein. After freezing, the cryoprobe is pulled out by a sharp jerky movement.
|
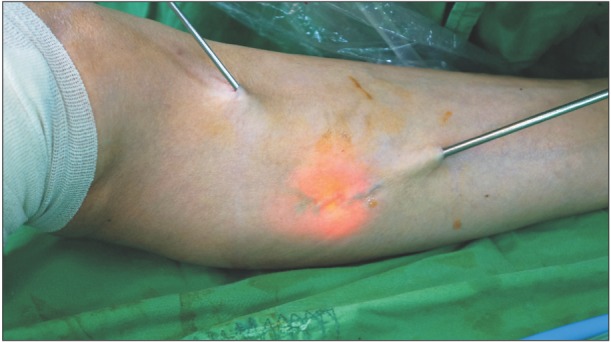 | Fig. 4The superficial varicosities is pulled out by slightly rotating the probe after freezing. Irrigationillumination device allows the operator to visualize the vein and accurate placement of additional stab incisions.
|
 | Fig. 5Picture of removed superficial varicosities.
|
Go to :

RESULTS
Eighty-four patients (131 limbs) were included in this study (37 males, 47 females). Forty-seven patients (56%) underwent surgery for both legs, whereas 37 patients (44%) required surgery for one leg. The mean age of patients was 53.3 years. The mean operative time for both legs was 64.7 minutes (range, 25–120 minutes). The mean operative time for one leg was 44 minutes (range, 15–70 minutes). In the EAP (Etiology-Anatomy-Pathophysiology) part of the CEAP classification, all patients belong to the Ep (primary), As (superficial), and Pr (reflux) categories. The preoperative symptoms of patients and the clinical classifications are presented in
Table 1. The most common symptom was heaviness (70%). Two patients (4 limbs) had unsightly varicosity without heaviness, leg cramp, edema, and pain despite obvious GSV reflux.
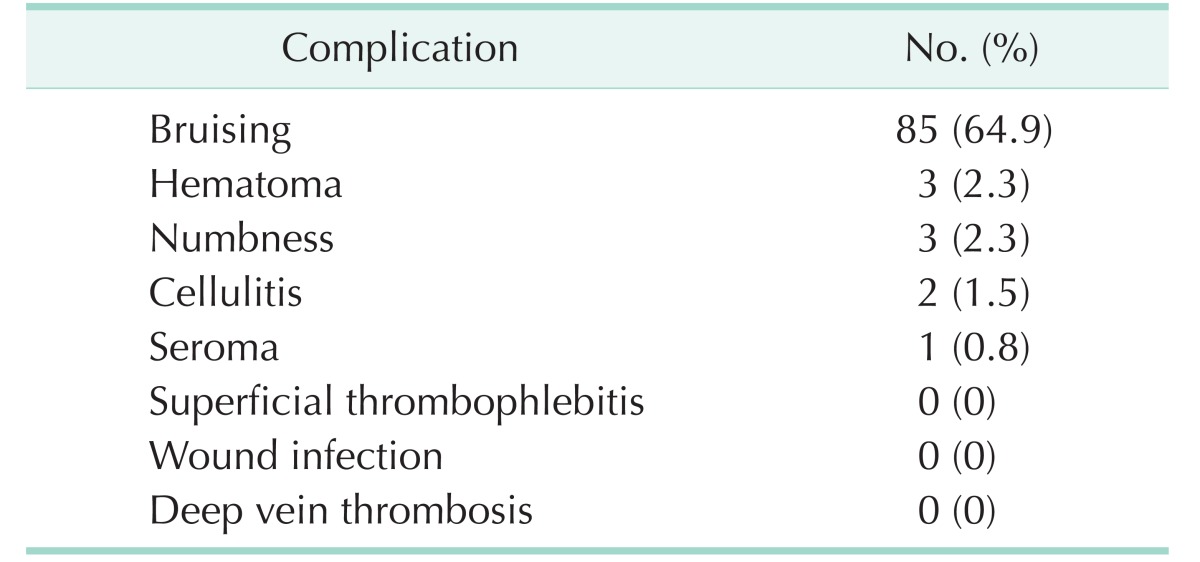
Table 2 summarizes the postoperative complications observed, including edema, cellulitis, superficial thrombophlebitis, cutaneous nerve damage, hematoma, and wound infection by postoperative week 2. Bruising was observed in 85 limbs (64.9%) at postoperative week 2 and was completely resolved at postoperative week 4 without further treatment. Hematomas were successfully treated with large bore needle aspiration or conservative treatment (3 limbs, 2.3%). Cutaneous nerve damage was defined as numbness and paresthesia and occurred in 3 limbs (2.3%). Seroma was noted in 1 limb (0.8%) and was treated with needle aspiration. The median number of incisions required for the operation was two (range, 1–5 incisions) and one incision at the level of the saphenous hiatus was required for the stripping of GSV.
Table 1
Preoperative demographic information (n = 131 limbs)
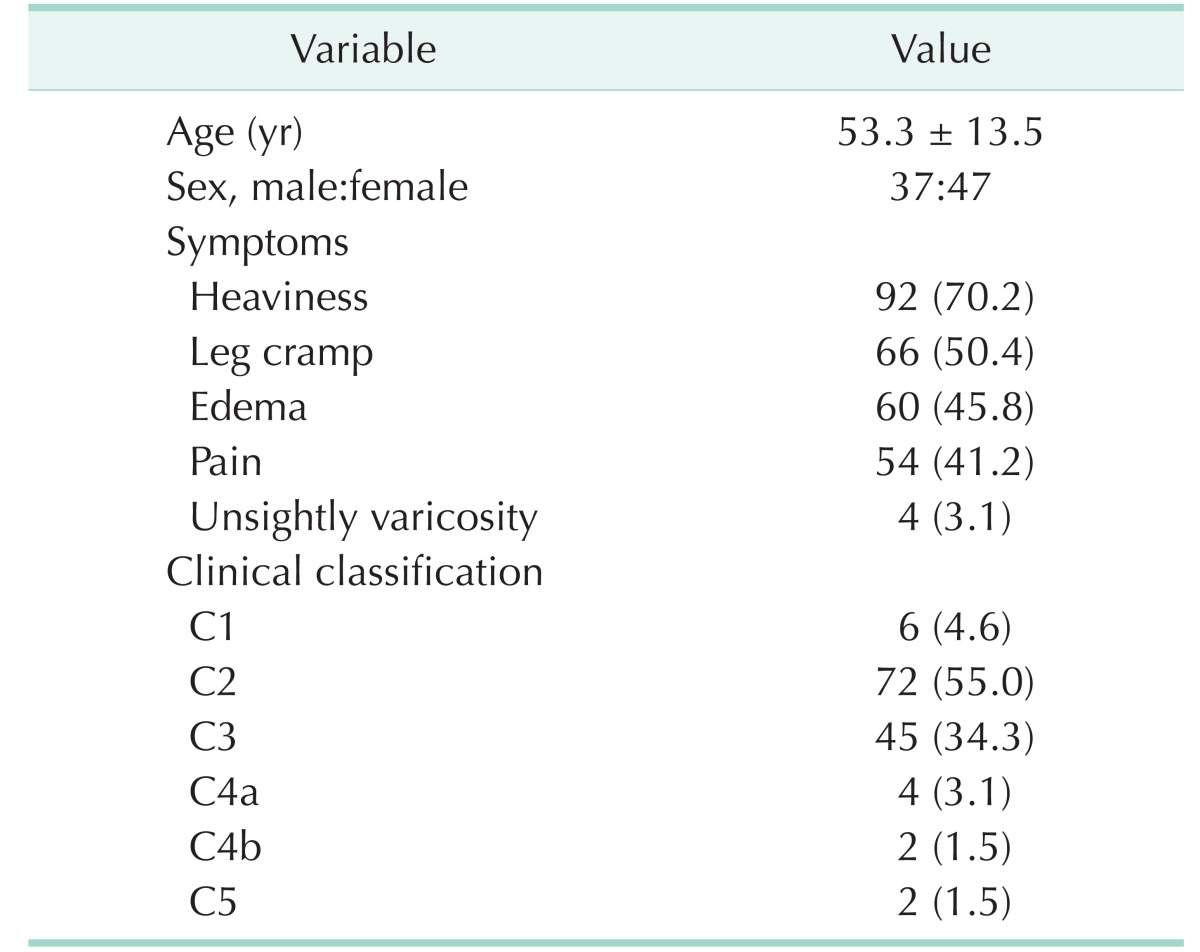

Table 2
Postoperative complications (n = 131 limbs)

Go to :

DISCUSSION
Lower extremity varicose vein is a common vascular disease and affects up to 40% of the general population [
6]. In the Edinburgh Vein study, 32% of females and 40% of males in a cohort of 1,566 randomly selected subjects had truncal varicosities [
7]. Previous studies have found out that the prevalence ranges from 20% to 25% in females and 10% to 15% in males [
8]. In our study, 47 female patients and 37 male patients had received operation and the gender differences in the current series were consistent with the differences reported in the previous reports. Patients increasingly want a perfect cosmetic outcome without recurrence after varicose vein operation. However, conventional stripping of GSV is not only time-consuming, but also requires multiple stab incisions. Furthermore, the conventional procedure may cause significant tissue trauma resulting in hematoma, and may lead to cutaneous nerve damage [
9]. Although minimally invasive endovenous techniques have proved their efficacy and good cosmetic results, several studies provide evidence that conventional GSV stripping results in excellent long-term outcome and reduces recurrence [
3]. Cryosurgery, which is similar to conventional stripping, consists of saphenofemoral junction ligation and GSV removal. This technique requires a small incision in the inguinal area, but does not require additional incisions above the knee for stripping of GSV, which are required in conventional stripping to fix the GSV before extraction. Cryosurgery could have good results regarding the recurrence rates because of its similarity to conventional stripping and no potential risk of recanalization, which is innate in endovenous laser or radiofrequency ablation. In the present study, the mean follow-up period was 13.5 months (range, 3–28 months). No recurrence was reported, although the follow-up period was not long.
Varicosities are a serious cosmetic defect and are particularly noticeable in female patients. To achieve a satisfactory cosmesis, surgical treatment is expected to be not only radical but also aesthetic. It is of utmost importance for patients not only to have the unsightly varicosities removed but also to bear no visible surgical scar. However, in conventional stripping and endovenous ablation, multiple stab incision phlebectomy and sclerotherapy on multiple sites is needed. Usually, multiple stab incision phlebectomy involves a large number of tiny skin incisions on the lower extremities, and sclerotherapy has the potential risk of tissue necrosis and skin pigmentation due to a hemosiderin deposition [
3]. In cryosurgery, after stripping of GSV, removal of the remaining varicosities is performed through a small incision, which considerably reduces the number of skin incision. During operation for superficial varicosities, we used an irrigation-illumination device. This device facilitates visualization of the veins requiring treatment without preoperative marking and intraoperative sonography (
Fig. 4). Infused tumescent fluid that consists of 1 L of 0.9% normal saline with 40 mL of 1% lidocaine and 1.5 mL 1:1000 epinephrine assists in defining the operative plane via hydrodissection, provides local anesthesia, and aids in hemostasis. The amount of tumescent fluid used was less than 400 mL.
Complication after cryosurgery can vary from postoperative hematoma, bruising, cellulitis, superficial thrombophlebitis, wound infection, DVT and cutaneous nerve damage [
3410]. We did not encounter DVT, superficial thrombophlebitis, or wound infection. Cutaneous nerve damage occurred in 2.3% of cases, which is comparable with previous reports of endovenous treatment [
1112]. The numbness and paresthesia due to nerve damage was resolved within 2 months postoperatively in all patients. Another important unfortunate adverse event of varicose vein surgery is hematoma formation. However, the incidence rate varies widely according to the treatment modality [
31213]. The disparity in hematoma incidence rate is due to varying definitions of hematoma, which includes bruising, ecchymosis and hematoma. The current study defined hematoma as a pocket or localized collection of blood, usually in the liquid or semiliquid form, within the subcutaneous space, and ecchymosis and bruising was defined as the spread of blood under the skin in a thin layer. In our study, the incidence of hematoma and bruising was 2.3% and 64.9%, respectively, at 2 weeks postoperatively. Hematoma developed at the groin area in a patient who had an immediate aspirin medication from the first postoperative day due to coronary artery occlusive disease and was resolved completely at followup 6 weeks postoperatively. Mild bruising was seen in 64.9% of operated limbs at postoperative week 2 and was completely resolved within 4 weeks. Cellulitis (2 limbs) and seroma (1 limb) were successfully treated with conservative treatment and needle aspiration, respectively, within 1 week.
The limitations of the current study were related to the inherent bias involved with retrospective studies and included a lack of randomization or a control group. Standardized clinical assessment and a patient satisfaction system would have been useful in providing the efficacy of cryosurgery for varicose veins, but the present study lacked the components of a standardized evaluation system. The main strengths of the current study were the single surgeon experience with coherent protocol. Suggestions for future work include continuing follow-up of patients who received cryosurgery for varicose veins to determine the long-term complication and recurrence rates. Future comparisons with the present study and endovenous procedures (radiofrequency or laser ablation of GSV) combined with sclerotherapy to assess potential differences also could be beneficial.
In conclusion, cryosurgery for varicose veins is an alternative and promising surgical option to reduce operative time, the number of incisions, and patient morbidity, and to achieve the best cosmetic results. Although the results of the current study are promising, more long-term follow-ups and prospective randomized studies between other treatment modalities are needed to encourage the use of cryosurgery for varicose veins.
Go to :







 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download