Abstract
Purpose
The average rate of surgical site infections (SSIs) for laparoscopic cholecystectomy (LC) has been reported in the literature to be between 0.4% and 6.3%. Also, these recent reviews have concluded that a prophylactic antibiotics for elective LCs in low-risk patients is not useful, but there were no results in high-risk patients.
Methods
The aim of this study was to investigate the role of a single dose of first-generation cephalosporin as a prophylactic antibiotic for patients undergoing elective LC, regardless of patient risk. This randomized clinical trial was conducted from October 2013 to December 2014 by single surgeon at our hospital. Patients were randomized into two groups by following method. Odd-numbered patients (group A) received 1-g cefazolin intravenously within 30 minutes before incision, whereas even-numbered patients (group B) received normal saline intravenously instead of prophylactic antibiotics, with the aim of including 100 patients in each group. SSIs were recorded and compared between the groups.
Results
There were no differences in preoperative demographics and postoperative findings between the groups. There were no superficial and deep SSIs in either group, 9 cases of superficial seromas developed (4.5%) in the cohort: 4 in group A (4%) and 5 in group B (5%). There were no significant associations between SSIs and the use of prophylactic antibiotics in either group. Additionally, the high-risk group did not show a significantly increased rate of SSIs.
Go to : 
Laparoscopic cholecystectomy (LC) carries an extremely low rate of postoperative infection compared with open cholecystectomy [1]. The average rate of SSIs for LC has been reported in the literature to be between 0.4% and 6.3%, which is lower than rates reported for open cholecystectomy [123]
Unlike for open cholecystectomy, many investigators have suggested that antimicrobial prophylaxis is probably unnecessary for LC patients because the infection rate for LC is already low, and the use of prophylactic antibiotics does not decrease the rate of wound infections or other postoperative infection complications [145678]. Also, these recent meta-analyses and systematic reviews have concluded that a prophylactic antibiotics for elective LCs in low-risk patients is not useful, but there were no results in high-risk patients [9]. Despite these studies on the topic, many other surgeons still use and recommend the administration of prophylactic antibiotics for LC in low-risk patients. Generally, preoperative single-dose cefazolin as a prophylactic antibiotic has been recommended and widely used in clean-contaminated surgeries such as cholecystectomy and biliary surgery to reduce SSI [10].
Therefore, the aim of this study was to investigate the role of a single dose of first-generation cephalosporin as a prophylactic antibiotic for patients undergoing elective LC, regardless of patient risk.
Go to : 
This randomized clinical trial was conducted from October 2013 to December 2014 by single surgeon, who had performed more than 1,000 conventional three-port LCs at Dong-A University Hospital. Patients were randomized into 2 groups by following method. Odd-numbered (1 and subsequent 3, 5, 7, …) patients (group A) received 1-g cefazolin intravenously within 30 minutes before incision, whereas even-numbered (2, 4, 6, …) patients (group B) received normal saline intravenously instead of prophylactic antibiotics, with the aim of including 100 patients in each group.
The study was approved by the Institutional Review Board of Dong-A University Hospital (approval number: 2011-088). Informed consent was obtained preoperatively from all patients.
All patients undergoing elective LC were enrolled in this study. Other exclusion criteria were acute cholecystitis with fever higher than 38℃ (acute cholecystitis without fever has included this study), pregnancy, immune compromised status, evidence of cholangitis and/or biliary pancreatitis, and previous endoscopic retrograde cholangiopancreatography. High-risk patients were defined as those with an American Society of Anesthesiologists (ASA) physical status classification higher than III, those with evidence of diabetes mellitus, those with a body mass index higher than 30, or those older than 70 years [11]. High-risk patients were not excluded in this study.
In preparation of patient, we did not remove unless hair will interfere with operation. Also, the skin was prepared with an appropriate antiseptic agent. Either the antibiotics or normal saline was administered within 30 minutes before incision. LC was performed in all patients using a 3-port technique.
The patient was positioned in the supine position on the operating room table and was placed in a reverse Trendelenburg position with the left side down after abdominal access and insufflating of the abdomen. Under general anesthesia, pneumoperitoneum (12 mmHg) was established after an 11-mm port was placed through an 11-mm transumbilical incision by the open method. A 10-mm 30-degree laparoscope was inserted through the umbilical port. Placement of 5-mm trocars occurred in the epigastric and right subcostal areas. A 5-mm Hem-o-Lok was typically used to ligate the cystic artery and duct after the gallbladder was freed from the liver bed with an electric spatula cautery. If gallbladder rupture and spill of bile or stones was encountered, the spilled stones were retrieved whenever possible, and peritoneal irrigation with saline was performed. A retrieval bag was then inserted blindly through the umbilical port, and the laparoscope was inserted through the umbilical port. The gallbladder was put into the retrieval bag using the remaining 2 ports and extracted through the umbilical port.
The postoperative course was monitored, and any incident, such as fever, wound infection, or intra-abdominal collection of abnormal fluid, was recorded. After discharge, the patients visited the hospital only once to be checked for SSIs. If no specific problems were found at this time, the patients did not require any further hospital visits. However, if abnormal symptoms were noted, the patients were asked to revisit the hospital.
Postoperative SSIs were defined according to the definitions of the Centers for Disease Control and Prevention [10] Generally, superficial or deep SSIs were defined as purulent discharge from the surgical site.
Patient demographics and clinical characteristics, including gallbladder perforation and bile spillage during LC, were recorded.
Excel (Microsoft, Redmond, WA, USA) and SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA) were used to analyze the data. Statistical significance was accepted for P-values of <0.050.
Go to : 
During the study period, 263 LCs were performed out of which 63 were excluded. Among these 63 cases, 42 were had acute cholesystitis with fever higher than 38℃, 13 had undertaken endoscopic retrograde cholangiopancreatography before LC, 4 had evidence of cholangitis and/or biliary pancreatitis, 1 was open conversion case, and 3 patients did not want to participate in this study.
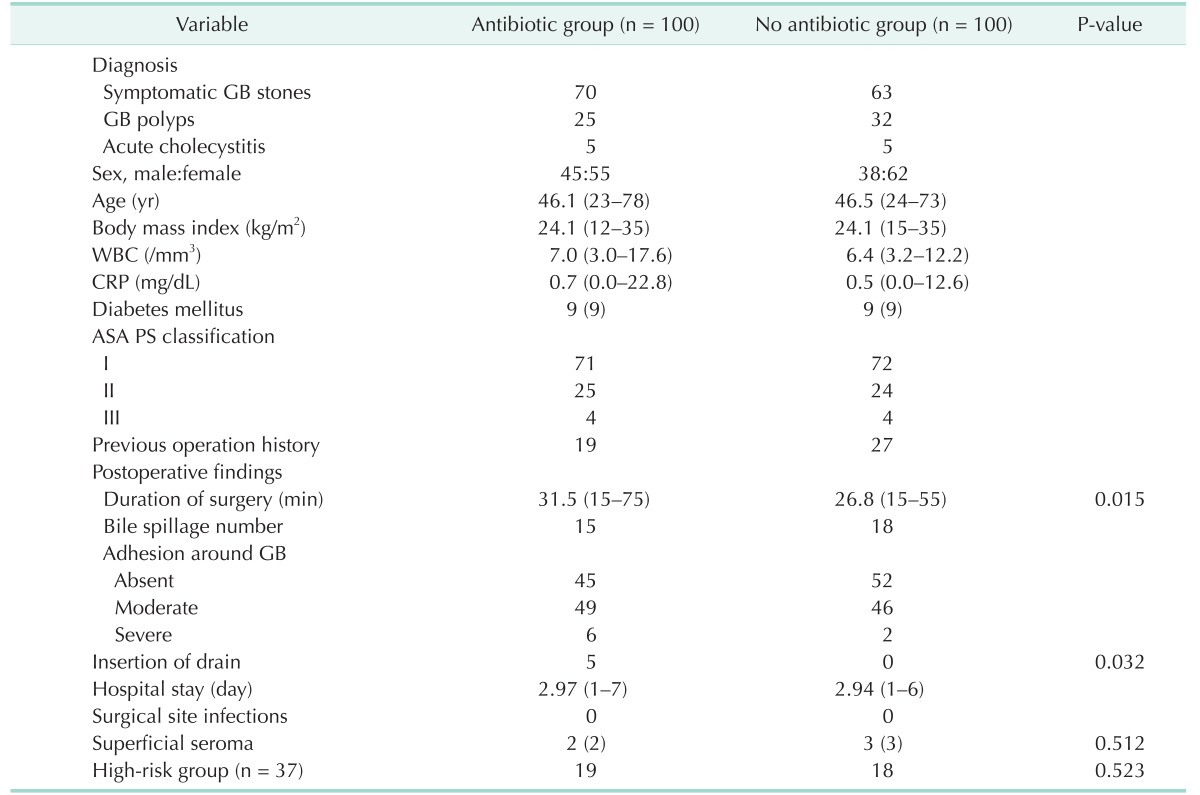
Groups A and B each included 100 patients. The groups had comparable pre- and postoperative findings in Table 1. No significant differences existed between the groups regarding sex, age, body mass index, laboratory findings, diabetes mellitus, ASA physical status classification, bile spillage, degree of adhesion, hospital stay, the number of high-risk patients, seroma or superficial SSIs. In total, 37 high-risk patients (18.5% of the cohort) were included: 19 in group A (19%) and 18 in group B (18%).
There were no superficial and deep SSIs in either group, superficial serosanguinous discharges (superficial seroma) were treated by simple drainage without per-oral antibiotics were not included in superficial SSIs. Nine cases of superficial seromas developed (4.5%) in the cohort: 4 in group A (4%) and 5 in group B (5%).
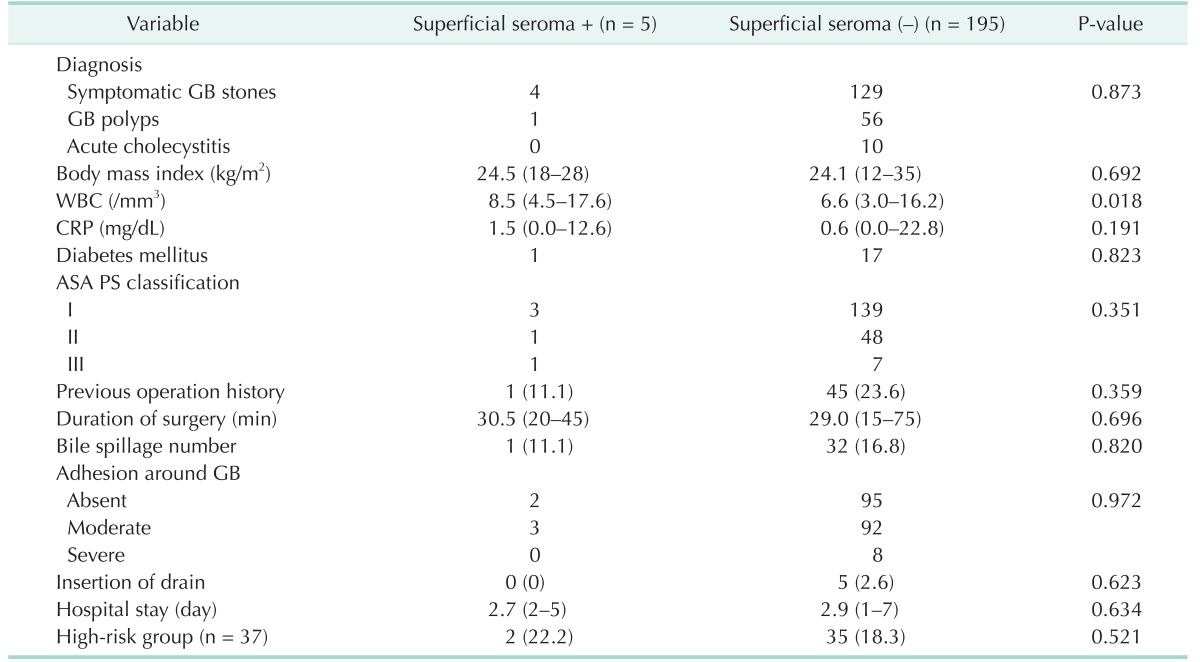
Because surgical site infection did not occur in this study, Table 2 compares the pre- and postoperative findings in the superficial seroma positive (+) and negative (–) groups. As shown in Table 2, WBC counts were the only significant preoperative findings associated with the occurrence of superficial seromas (P = 0.018). The other recorded pre- and postoperative findings had no statistically significant associations with the occurrence of superficial seromas (P > 0.050). Additionally, there was no difference in the number of superficial seromas in the high-risk patients.
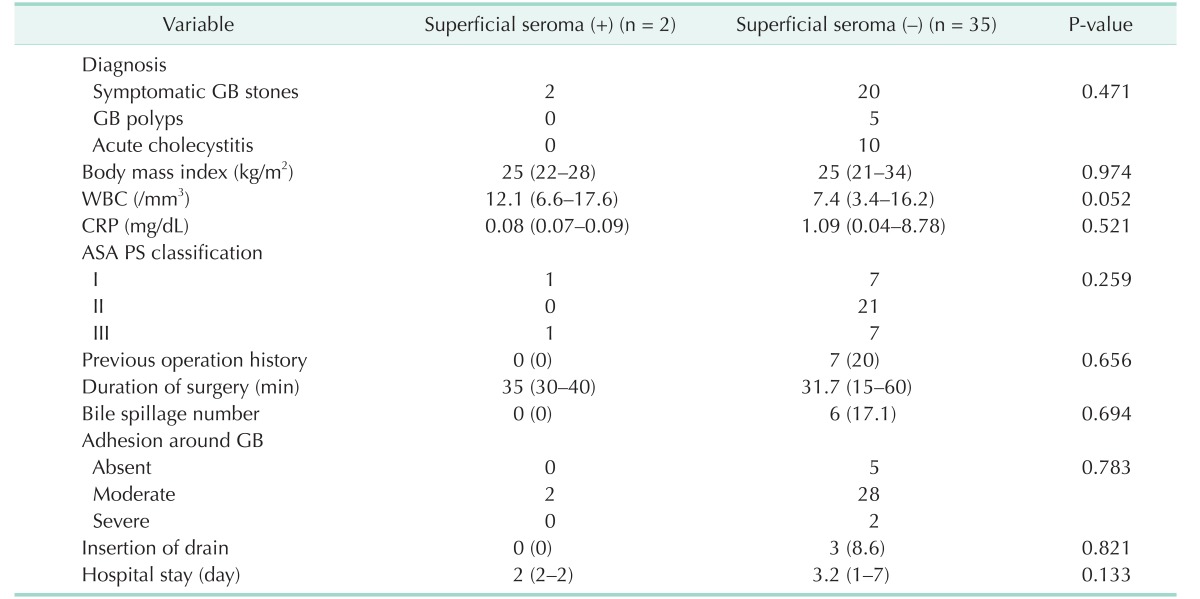
The present study also performed a univariate analysis of high-risk patients. Table 3 indicates the associations between the pre- and postoperative findings and the occurrence of superficial seromas in the high-risk patients. As shown in Table 3, WBC count was the only factor related to the occurrence of superficial seromas (P = 0.052), and none of the other recorded factors had a statistically significant association with superficial seromas occurrence in the high-risk patients (P > 0.050).
Go to : 
Ideally, prophylactic antibiotics should prevent SSIs as well as SSI-related morbidity and mortality and reduce the duration and cost of health care (when the costs associated with the management of SSI are considered, the cost-effectiveness of prophylaxis becomes evident). However, the incorrect use of prophylactic antibiotics can increase antibiotic resistance and the cost of health care. Therefore, appropriate selection of prophylactic antibiotics has medical benefit and significance. Comparisons of first-generation cephalosporin with second- or third-generation agents as prophylactic antibiotics have revealed no significant difference in efficacy among the agents [11].
In our country, the guideline for the use of prophylactic antibiotics is that a single dose of first-generation cephalosporin is administered within 1 hour prior to skin incision. Despite this guideline, some surgeons still use antibiotics such as second-generation or higher cephalosporin or combinations of other antibiotics in our country [12].
In our study, there was no SSIs and no significant difference (P = 0.523) between the superficial seroma rates in groups A and B (4% and 5%, respectively). These results indicate that the administration of a single dose of a prophylactic antibiotic failed to decrease the likelihood of SSIs after LC. These findings are concordant with previous studies showing no correlations between SSI rate and whether prophylactic antibodies had been administered [5678131415].
Numerous meta-analyses and cohort studies showed that overall infection rates after LC range from 0% to approximately 4% in patients not receiving antimicrobial prophylaxis and from 0% to 7% in those receiving prophylaxis [11]. However, these studies have generally focused on low-risk, elective LC patients. The high-risk factors associated with postoperative SSIs after biliary procedures, diabetes, long procedure duration (over 120 minutes), intraoperative gallbladder rupture, age >70 years, conversion of laparoscopic to open cholecystectomy, high ASA physical status classification (≥3), episode of biliary colic within 30 days before the procedure, bile spillage, jaundice, pregnancy, nonfunctioning gallbladder, and immunosuppression [111617]. Obesity (a BMI of >30 kg/m2) was also found to be a risk factor in some studies [11]. Therefore, the administration of prophylactic antibiotics is recommended for these high-risk patients.
The most unique aspect of our study was the inclusion of high-risk patients in both groups. In addition, patients with bile spillage (intraoperative gallbladder perforation) were classified as low risk because it was not possible to preoperatively identify gallbladder perforation in a prospective study. Many studies have reported that the use of prophylactic antibiotics significantly reduces the occurrence of positive bile culture, resulting in a significant reduction in complications induced by postoperative infections. Additionally, Dervisoglou et al. [18] mentioned that both positive bile culture and intraoperative gallbladder rupture were strongly associated with the development of SSI. In contrast, Uludag et al. [16] stated that overall SSI rate did not correlate with the presence of bacteria in the bile or with gallbladder rupture. Therefore, the effects of bile or stone spillage due to perioperative gallbladder perforation on the occurrence of SSI remains controversial.
Although bile culture was not performed in the present study, no relationship was found between bile spillage and SSI occurrence (P = 0.820). We did not perform bile culture because this method does not guarantee the preoperative identification of which patients have bactibilia. We believe that routine plastic endo-bag use, local peritoneal irrigation and adequately control wound contamination in the presence of gallbladder rupture are effective in preventing SSIs. Therefore, Bile spillage or stone spillage due to perioperative gallbladder perforation could be classified as a low-risk group.
In our study, 37 patients (18.5%) were considered high risk: 19 in group A (19%) and 18 in group B (18%). Although the sample size was small, according to our study, there were no SSIs in both group, the number of superficial seroma positive high-risk patients was only 2 (22.2%), whereas there were 35 superficial seroma negative high-risk patients (18.3%) (P = 0.521). Because our results indicated that the use of a single dose of firstgeneration cephalosporin in high-risk patients does not seem to affect the incidence of SSIs, the use of prophylactic antibiotics is not necessary for these patients. Because of the limitation of small sample size in this study, to assess the evaluation of prophylactic antibiotics use in high-risk patients, multicenter randomized controlled trials are needed.
The only statistically significant risk factor for superficial seromas in all patients (Table 2) (P = 0.018) and in the high-risk group (Table 3) (P = 0.052) was preoperative WBC count. In all patients and in the high-risk group, the mean WBC counts for superficial seroma positive patients were 8,500/mm3 and 12,100/mm3, respectively. Few studies have reported that WBC count serves as a risk factor for SSIs [1119].
Generally, increased WBC counts may reflect the possibility of complicated gallbladder disease. Although there were no SSIs in our study, increased WBC count may be used as a screening factor for the administration of prophylactic antibiotics. If WBC count is higher than the accepted upper limit (10,000/mm3) preoperatively, this parameter should be carefully considered when deciding on the use of prophylactic antibiotics.
Since most trials conducted to date have focused only on low-risk patients, the inclusion of high-risk patients in this study for the evaluation of prophylactic antibiotic use in elective LC is unique compared to other studies. However, because our study was conducted in single center and the sample size was not large, our findings could not be generalized to both low-risk and high-risk patients who undergo elective LC. Therefore, our results can be serve as a basis for large scale studies.
Although already well known, to prevent SSIs, we think appropriate preparation of patient (Do not remove unless hair will interfere with the operation; if hair removal is necessary, remove by clipping and do not use razors), surgical scrubbing, surgical site skin preparation, meticulous surgical skill and short operation time (mean 29.1 minutes in our study) are more important than using prophylactic antibiotics in elective clean LC.
In conclusion, based on our data, there was no postoperative SSIs and no difference in the rate of postoperative seromas for patients undergoing elective LC regardless of the use of prophylactic antibiotics, even though in high risk patients. Our study can conclude that there is no need to use pro prophylactic antibiotics in low-risk patients, but in deciding whether to use prophylactic antibiotics in high-risk patients, further studies are needed.
Go to : 
References
1. Chang WT, Lee KT, Chuang SC, Wang SN, Kuo KK, Chen JS, et al. The impact of prophylactic antibiotics on postoperative infection complication in elective laparoscopic cholecystectomy: a prospective randomized study. Am J Surg. 2006; 191:721–725. PMID: 16720138.

2. Biscione FM, Couto RC, Pedrosa TM, Neto MC. Comparison of the risk of surgical site infection after laparoscopic cholecystectomy and open cholecystectomy. Infect Control Hosp Epidemiol. 2007; 28:1103–1106. PMID: 17932836.
3. McGuckin M, Shea JA, Schwartz JS. Infection and antimicrobial use in laparoscopic cholecystectomy. Infect Control Hosp Epidemiol. 1999; 20:624–626. PMID: 10501264.

4. Koc M, Zulfikaroglu B, Kece C, Ozalp N. A prospective randomized study of prophylactic antibiotics in elective laparoscopic cholecystectomy. Surg Endosc. 2003; 17:1716–1718. PMID: 12802644.

5. Mahatharadol V. A reevaluation of antibiotic prophylaxis in laparoscopic cholecystectomy: a randomized controlled trial. J Med Assoc Thai. 2001; 84:105–108. PMID: 11281486.
6. Yildiz B, Abbasoglu O, Tirnaksiz B, Hamaloglu E, Ozdemir A, Sayek I. Determinants of postoperative infection after laparoscopic cholecystectomy. Hepatogastroenterology. 2009; 56:589–592. PMID: 19621660.
7. Higgins A, London J, Charland S, Ratzer E, Clark J, Haun W, et al. Prophylactic antibiotics for elective laparoscopic cholecystectomy: are they necessary? Arch Surg. 1999; 134:611–613. PMID: 10367869.
8. Sharma N, Garg PK, Hadke NS, Choudhary D. Role of prophylactic antibiotics in laparoscopic cholecystectomy and risk factors for surgical site infection: a randomized controlled trial. Surg Infect (Larchmt). 2010; 11:367–370. PMID: 20575704.

9. Ruangsin S, Laohawiriyakamol S, Sunpaweravong S, Mahattanobon S. The efficacy of cefazolin in reducing surgical site infection in laparoscopic cholecystectomy: a prospective randomized double-blind controlled trial. Surg Endosc. 2015; 29:874–881. PMID: 25052130.

10. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Guideline for prevention of surgical site infection, 1999. Am J Infect Control. 1999; 27:97–132. PMID: 10196487.

11. Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013; 70:195–283. PMID: 23327981.

12. Nam EY, Kim HB, Bae H, Moon S, Na SH, Kim SY, et al. Appropriateness of surgical antibiotic prophylaxis in a tertiary hospital. Korean J Nosocomial Infect Control. 2014; 19:64–70.

13. Kacelnik O, Alberg T, Mjaland O, Eriksen H, Skjeldestad FE. Guidelines for antibiotic prophylaxis of cholecystectomies in Norwegian hospitals. Surg Infect (Larchmt). 2013; 14:188–191. PMID: 23530809.

14. Kuthe SA, Kaman L, Verma GR, Singh R. Evaluation of the role of prophylactic antibiotics in elective laparoscopic cholecystectomy: a prospective randomized trial. Trop Gastroenterol. 2006; 27:54–57. PMID: 16910066.
15. Dobay KJ, Freier DT, Albear P. The absent role of prophylactic antibiotics in low-risk patients undergoing laparoscopic cholecystectomy. Am Surg. 1999; 65:226–228. PMID: 10075297.
16. Uludag M, Yetkin G, Citgez B. The role of prophylactic antibiotics in elective laparoscopic cholecystectomy. JSLS. 2009; 13:337–341. PMID: 19793473.
17. Yan RC, Shen SQ, Chen ZB, Lin FS, Riley J. The role of prophylactic antibiotics in laparoscopic cholecystectomy in preventing postoperative infection: a meta-analysis. J Laparoendosc Adv Surg Tech A. 2011; 21:301–306. PMID: 21443433.

18. Dervisoglou A, Tsiodras S, Kanellakopoulou K, Pinis S, Galanakis N, Pierakakis S, et al. The value of chemoprophylaxis against Enterococcus species in elective cholecystectomy: a randomized study of cefuroxime vs ampicillin-sulbactam. Arch Surg. 2006; 141:1162–1167. PMID: 17178957.
19. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999; 20:250–278. PMID: 10219875.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download