Abstract
Purpose
Few studies about laparoscopic surgery for splenic flexure colon cancer have been published. This study aims to compare the short- and long-term outcomes of laparoscopic surgery for splenic flexure colon cancer with those of conventional open surgery.
Methods
From January 2004 to December 2010, 51 consecutive patients who underwent curative resection for stages I–III splenic flexure colon cancer were enrolled. Thirty-three patients underwent laparoscopy-assisted colectomy, while 18 patients underwent conventional open colectomy. Short- and long-term outcomes of the 2 groups were compared.
Results
There were no differences in baseline characteristics, intra- and postoperative complications. The laparoscopy group showed longer operation time (median [interquartile range, IQR]: 295.0 [255.0–362.5] minutes vs. 180.0 [168.8–206.3] minutes, P < 0.001). In the laparoscopy group, return of bowel function was faster (median [IQR]: 3 [2–4] vs. 4 [3–5], P = 0.007) and postoperative hospital stay was shorter (median [IQR]: 9 [8–11] vs. 10.5 [9–19], P = 0.026). There were no statistically significant differences in overall survival rate (84.3% vs. 76.0%, P = 0.560) or disease-free survival rate (93.8% vs. 74.5%, P = 0.078) between the 2 groups.
The safety and efficacy of laparoscopic surgery for colorectal cancer has been confirmed by several randomized clinical trials [1234]. It provides less pain, quicker recovery of bowel movement, and shorter hospital stay while no significant difference in long-term survival has been shown compared to open surgery. Laparoscopic surgery for colorectal cancer is steadily increasing and becoming a primary treatment modality in most large-volume centers.
Nevertheless, splenic flexure colon cancer has been excluded in most of studies of laparoscopic surgery. It is because the incidence of cancer in this region is low among total colorectal cancers, and laparoscopic skills for dissecting main lymph nodes with mobilizing colon are technically demanding. These procedural difficulties can increase intra- and postoperative complications, and lead to inferior survival outcome compared to that of open surgery.
Studies of laparoscopic surgery for splenic flexure colon cancer are sparse and all reports to date have been retrospective. In particular, there have been no reports comparing long-term oncologic outcomes between laparoscopic and open surgery for splenic flexure colon cancer. The aim of this study was to compare the short- and long-term outcomes of laparoscopic surgery for splenic flexure colon cancer with those of conventional open surgery.
Splenic flexure colon cancer was defined as cancer arising from the portion of the colon between the distal third of the transverse colon and the proximal part of the descending colon within 10 cm of the splenic flexure edge. Medical records of 68 consecutive patients who underwent curative resection for pathologically proven primary splenic flexure colon adenocarcinoma performed by 3 surgeons in 2 hospitals between January 2004 and December 2010 were retrospectively reviewed. Patients were excluded for the following reasons: stage 0 (n = 2) or IV (n = 3), emergency surgery (n = 4), another malignant disease within 5 years before or after surgery (n = 3), synchronous colon cancer (n = 3), or alteration of normal colon anatomy due to previous colorectal surgery (n = 2). The remaining 51 patients were included in the analysis. This study was approved by the Institutional Review Board of the Catholic Medical Center (approval number: XC15RIMI0095K).
All operations were performed by 3 colorectal surgeons. The first surgeon performed laparoscopy-assisted colectomy (LAC) for all stages of colon cancer except those that were suspected stage T4 on preoperative evaluation. The second surgeon performed conventional open colectomy (OC) only. The third surgeon performed laparoscopic surgery during his residency for the first time. He performed open surgery early in his career as a colorectal surgeon, but now laparoscopic surgery is his primary treatment modality for colorectal disease. His open surgery was influenced by the second surgeon, and his laparoscopic surgery was influenced by the first.
The 2 hospitals in this study are affiliated to one institution, the Catholic University of Korea, and this institution has traditionally emphasized “plane surgery” as a surgical principle in training programs. Therefore, all surgeons in this study have mastered this principle not only in open surgery, but also in laparoscopic surgery. They have been able to dissect the visceral fascia layer from the retroperitoneal plane without compromising the fascia layer of tumor-bearing tissue during surgery. This concept is quite similar to the recent technique of complete mesocolic excision [5].
Histology was confirmed by colonoscopic biopsy prior to surgery for all patients. A barium enema and/or abdomino-pelvic CT were administered. For patients scheduled for laparoscopic surgery and clinically estimated as T2 or less by preoperative studies, colonoscopic tattooing and clipping was performed before surgery to localize the lesion intracorporeally. Oral mechanical bowel preparation and perioperative intravenous antibiotics were prescribed to all patients. Fluorouracil-based adjuvant chemotherapy was administered based on pathology and surgeon judgment.
Both patient arms were placed along the body and the Trendelenburg position with right tilting was adopted. A medial-to-lateral approach was used to dissect the mesocolon. Dissection began with opening the visceral peritoneum along the border of the inferior mesenteric artery (IMA). High ligation of the IMA was performed if there was suspected local lymph node metastasis; otherwise, the origin of the left colic artery (LCA) was identified and ligated after IMA skeletonization. The inferior mesenteric vein (IMV) was delineated and the left colic vein (LCV) was identified. Ligation and division of the LCV were performed just before it drained to the IMV, or the IMV was ligated around the lower border of the pancreas instead. The plane between the mesocolon and retroperitoneum was dissected, along with the Toldt fascia, to the lower border of the pancreas. Then, the lateral detachment of the descending colon was performed.
The transverse mesocolon was retracted to appear unfolded like a fan. The middle colic vessels were identified along the lower border of pancreas facing toward the duodenal c loop. Lymph node dissection was initiated from the root of the middle colic vessels, and the right and left branches of the middle colic vessels were identified. Ligation and division were made at the origin of the middle colic vessels or the left branch, depending on clinical judgment.
The anterior approach [6] was mainly used for splenic flexure mobilization. In this approach, the position was changed to reverse Trendelenburg with sustained right tilting. The omentum was dissected from the transverse colon, located about 10 cm from the tumor. After the lesser sac was approached, omental dissection was continued to the most cranial side along the gastroepiploic vessels while ensuring their preservation. The omentum and splenocolic ligament were divided from the spleen, and splenic flexure mobilization was completed with the separation of the mesocolon from the pancreas. Mesocolon division was finalized by securing sufficient proximal and distal resection margins. The tumorbearing segment was extracted through the mini-laparotomy site. End-to-side fashion using staplers or end-to-end fashion hand sewn extracorporeal anastomosis was made (Fig. 1).
The principles of plane surgery and the medial-to-lateral approach were maintained in open surgery. Midline or left subcostal incisions were used, and anastomosis was performed in the same fashion. Unlike in laparoscopic surgery, the left branch of the middle colic vessels was ligated and divided without checking the origin or left branch of the middle colic vessels when the lesion was suspected to be an early tumor.
Patients visited the outpatient clinic within a month after surgery, and then follow-up was conducted every 3 months for a duration of 2 years, every 6 months between 2 and 5 years after surgery, and yearly thereafter. History, physical examination, and basic blood tests with serum CEA level were checked at every visit. Colonoscopy and abdomino-pelvic CT were performed at least annually after the surgery. Additional tests, such as chest CT or positron emission tomography CT, were performed when clinically necessary. The cutoff time for last follow-up was December 2014.
The following variables were measured: baseline characteristics, intra- and postoperative (within 30 days after surgery or during the same admission period) complications, postoperative recovery course, pathologic characteristics for the oncologic quality of the resected specimen, 5-year disease-free survival (DFS), and 5-year overall survival (OS).
Expression of the median (interquartile range) and the Mann-Whitney U-test were used for continuous variables. Fisher exact test was used to compare categorical data. The Kaplan-Meier method was performed to calculate survival analysis, and survival comparisons were performed with the log-rank test. Statistical significance was defined as P < 0.05. IBM SPSS ver. 18.0 (IBM Co., Armonk, NY, USA) was used for statistical analysis.
Among the 51 enrolled patients, 33 belonged to the LAC group and 18 belonged to the OC group. All patients of the first surgeon (n = 29) underwent LAC and all patients of the second surgeon (n = 15) underwent OC. Seven patients underwent surgery performed by the third surgeon, with 4 undergoing LAC and 3 undergoing OC.
There were no significant differences in age, sex, body mass index, American Society of Anesthesiologists physical status classification, history of previous abdominal surgery, or preoperative CEA level between the 2 groups (Table 1). Two patients in the LAC group underwent subtotal colectomy, which was defined as the resection of more than 2 segments of colon, to have extended resections with sufficient margin. However, they were not converted to open surgery and there was no statistical difference in operative methods. The rate of adjuvant chemotherapy was not significantly different between the groups (81.8% vs. 66.7%, P = 0.172), and there was no synchronous resection of other intra-abdominal organs.
There were no statistical differences in pathological stage according to the Union for International Cancer Control 6th edition, including pathologic T stage, pathologic N stage, histologic differentiation (Table 2). The distal resection margin was significantly greater in the LAC group (median [interquartile range, IQR]: 13.0 [10.5–19.5] cm vs. 8.6 [7.0–11.0] cm, P = 0.001), and the proximal resection margin also showed a tendency toward increased length in the LAC group (median [IQR]: 11.5 [9.3–15.3] vs. 8.9 [5.8–12.6], P = 0.068). Despite the difference in resection margin, the number of retrieved lymph nodes revealed no difference (median [IQR]: 15.0 [6.0–24.5] vs. 17.0 [8.8–22.5], P = 0.820).
The LAC group showed significantly longer operating time than the OC group (median [IQR]: 295.0 [255.0–362.5] minutes vs. 180.0 [168.8–206.3] minutes, P < 0.001) (Table 3). There were 3 cases of intraoperative complications in the LAC group, though the difference was not significant (9.1% vs. 0.0%, P = 0.544). Complications included a small bowel serosal injury, which was resolved by intracorporeal primary repair, and 2 splenic injuries. Intracorporeal bleeding control and laparoscopic splenectomy were needed, respectively.
There were no differences in postoperative complications between the 2 groups (18.2% vs. 33.3%, P = 0.304). An anastomotic leak occurred once in the OC group, leading to reoperation (irrigation and loop ileostomy formation), which was classified as grade IIIb according to the Clavien-Dindo classification system [7]. The remaining 11 cases were all categorized as grade I or II, and resolved without any sequela. There was also no difference in the grade distribution of Clavien-Dindo classification between groups (P = 0.279). Two laparoscopic surgeries (6.1%) were converted to open surgery because of the inability to identify accurate anatomical structure due to anomalies and because of anastomotic site twisting, respectively. Regarding postoperative recovery, there was no difference in time to diet resumption (median [IQR]: 4 [4–4.5] days vs. 4 [4–5] days, P = 0.729), but flatus passing was faster in the LAC group (median [IQR]: 3 [2–4] days vs. 4 [3–5] days, P = 0.007). Postoperative hospital stay was shorter in the LAC group than the OC group (median [IQR]: 9 [8–11] days vs. 10.5 [9–19] days, P = 0.026).
The median follow-up period was 59.0 months (IQR, 50.0–73.5 months) for the LAC group, and 61.0 months (IQR, 27.8–87.0 months) for the OC group (P = 0.760). Comparison of survival outcomes for the stages I–III patients revealed increased survival rates in the LAC group, but there were no statistically significant differences in cumulative 5-year DFS rate (93.8% vs. 74.5%, P = 0.078) or cumulative 5-year OS rate (84.3% vs. 76.0%, P = 0.560) (Fig. 2). During the follow-up period, 2 patients in the LAC group and 4 patients in the OC group, all with stage III initial pathology, experienced recurrence. The patterns of recurrence and characteristics of patients with recurrence are summarized in Table 4. Five of 6 patients with recurrence died, and the other patient was lost to follow-up 87 months postoperatively. He had metastatic perirectal lymph nodes after primary surgery and received lymphadenectomy followed by chemotherapy. The 5-year DFS rate of stage III LAC patients was 84.6% (2 of 13).
Splenic flexure colon cancer accounts for approximately 5% of all colorectal cancers [89]. Clinical characteristics of this disease include higher prevalence in males, younger age at diagnosis, and more common presentation of obstruction [8].
There have been few studies about laparoscopic surgery for splenic flexure colon cancer due to its low incidence and the high technical skill required for operating in its location.
The splenic flexure colon receives dual blood supply from the LCA and the left branch of the middle colic artery [10]. Thus, identification and ligation of these 2 vessels at their origin with lymphadenectomy are mandatory for performing complete radical surgery in this area. However, such a procedure in close proximity to critical organs, like the pancreas and duodenum, carries significant risks.
In addition, full mobilization of the splenic flexure colon is needed to obtain tension-free anastomosis and a sufficient resection margin, yet the high anatomical position of the splenic flexure and omental adhesion make this difficult. Even highly experienced laparoscopic colorectal surgeons consider splenic flexure mobilization the most difficult procedure in their field according to the study of Jamali et al. [11] Akiyoshi et al. [12] performed multivariate analysis of left colon surgery and reported that splenic flexure mobilization was the most significant factor causing longer operative time, increased intraoperative complications, and higher volume of estimated blood loss.
For these reasons, splenic flexure colon cancer has been excluded from major randomized clinical trials. The only known reports on this condition pertain to the short-term safety of laparoscopic surgery [1314] and the comparison of short-term outcomes of laparoscopic splenic flexure colon cancer surgery to that of open surgery [15]. In particular, there are no known reports comparing long-term survival outcomes of laparoscopic surgery to open surgery for this lesion.
The present study compares laparoscopic and open surgery for splenic flexure colon cancer with data from 2 highly experienced surgeons (one specialized in open surgery and the other in laparoscopy) and a specialized young surgeon who trained under the influence of their surgery.
Our results regarding short-term outcomes were similar to previous studies about splenic flexure and those on other colon cancer lesions. Although average operative time increased by over an hour due to the technical difficulty of laparoscopic surgery, there were no differences in perioperative complications and patient recovery was faster, as shown by sooner resumption of bowel movement and shorter length of postoperative hospital stay. Nakashima et al. [15] compared short-term outcomes of laparoscopic and open surgery for splenic flexure colon cancer. They also reported longer operative times in the LAC group and better outcomes for flatulence, diet resumption, and postoperative hospital stay. Though the OC group in that study experienced higher estimated blood loss and more postoperative complications, these were likely due to a significantly higher T stage and larger tumor size.
Conversion to open surgery occurred in 2 of 33 patients (6.1%). Other reports of laparoscopic resection of splenic flexure colon cancer reported a conversion rate of 0%–6.3% [131415]. One reason for conversion in our study was anastomosis site twisting. Pisani Ceretti et al. [13] suggested that intracorporeal anastomosis may prevent this, so further research on the optimal method of anastomosis is necessary.
There were no differences in the long-term survival outcome between the LAC and OC groups, in concordance with major studies on other colon cancer lesion sites. The survival curves of both DFS and OS revealed a tendency toward superior survival for the LAC group, but this is thought to be due to a significantly higher rate of lymphatic and perineural invasion in the OC group.
Two patients (6.06%) in the stages I–III LAC group experienced recurrence in the follow-up period (median, 59 months). When compared to other studies of laparoscopic surgery for colon cancer of the same stages, reporting 2 recurrences out of 23 patients (8.70%) during a mean of 33 months of follow-up in one report [13], and 2 of 11 patients (18.18%) during a median of 28.7 month of follow-up in other report [14], our study showed a superior outcome.
To our knowledge, this is the first report revealing that longterm oncologic outcomes of laparoscopic surgery for stages I–III splenic flexure colon cancer are comparable to those of open. However, there are some issues that must be considered when interpreting these results.
First, this study was designed to compare the outcomes of laparoscopic surgery to those of conventional open surgery. These different approaches were typically performed by different surgeons. Our laparoscopic data were almost entirely from the first surgeon, and open surgery data were almost entirely from the second. To most accurately compare laparoscopy and open surgery, data must be obtained via randomized assignment to one of these surgical methods. However, performing a randomized controlled trial is difficult because there are few cases of splenic flexure colon cancer. In addition, a retrospective study comparing data from surgeons who can do both types of surgery would also be difficult because the indications for the different types of surgery lead to significant variation in the basic characteristics of the groups Hence, this study sought to compare the outcomes of both procedures indirectly by analyzing data from different surgeons.
This resulted in variation in results that originated from the different operative principles of the surgeons, not from different types of surgery. For example, the results of the present study revealed significantly longer distal margins in the LAC group, and similar tendencies in the proximal margin. This seems to be related to the surgical disposition of the practitioner rather than the difference between the LAC and OC groups. Ideally, comparisons should be made between groups with similar basic characteristics, and this is an inherent limitation of the retrospective design and small population of this study. The appropriate resection range for splenic flexure colon cancer has not yet been established [13], and the length of the resection margin in previous studies was about 6–10 cm [1315]. With median proximal and distal resection margins of 8.9 and 8.6 cm, respectively, in the OC group, the OC in this study seems acceptable as a conventional open surgery for splenic flexure colon cancer.
The results of this study may also be biased by the learning curve of the third surgeon for laparoscopic colectomy. However, it seems unlikely that the learning curve affected surgery outcomes considering the comparable complication rates and superior postoperative recovery of the LAC group. Operating time was still significantly longer for the LAC group even after excluding operations by the third surgeon (median [IQR]: 300 [275–390] minutes vs. 180 [170–205] minutes, P < 0.001). This suggests that the longer operating time of the LAC group could have originated from the technical difficulty difference between open and laparoscopic surgery for managing splenic flexure colon cancer rather than the learning curve.
In conclusion, laparoscopic surgery for splenic flexure colon cancer is advantageous for patient recovery compared to open surgery and is comparable based on safety and long-term survival. Experienced surgeons and deliberate patient selection make this a good alternative to open surgery.
Figures and Tables
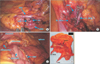 | Fig. 1(A) Descriptive pictures of the operative procedure. (A) Left colic artery (LCA) was identified and skeletonized before being ligated at its origin. (B) Left branch of the middle colic artery (MCA) was identified and clipped before division. (C) Laparoscopic view after finishing splenic flexure mobilization. (D) Specimen of colon after laparoscopic left hemicolectomy. Preoperative tattooing and clipping were done for this specific patient. IMV, inferior mesenteric vein; IMA, inferior mesenteric artery; MCV, middle colic vein. |
 | Fig. 2Cummulative survival of stages I–III splenic flexure colon cancer patients. There is no significant difference between the laparoscopy-assisted colectomy, (LAC; n = 33) and open colectomy (OC; n = 18) groups. (A) Cummulative 5-year overall survival (OS) rate (84.3% vs. 76.0%, P = 0.560). (B) Cummulative 5-year disease-free survival (DFS) rate (93.8% vs. 74.5%, P = 0.078). |
References
1. Fleshman J, Sargent DJ, Green E, Anvari M, Stryker SJ, Beart RW Jr, et al. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann Surg. 2007; 246:655–662.
2. Green BL, Marshall HC, Collinson F, Quirke P, Guillou P, Jayne DG, et al. Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg. 2013; 100:75–82.
3. Colon Cancer Laparoscopic or Open Resection Study Group. Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009; 10:44–52.
4. Lacy AM, Delgado S, Castells A, Prins HA, Arroyo V, Ibarzabal A, et al. The long-term results of a randomized clinical trial of laparoscopy-assisted versus open surgery for colon cancer. Ann Surg. 2008; 248:1–7.
5. Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation--technical notes and outcome. Colorectal Dis. 2009; 11:354–364.
6. Benseler V, Hornung M, Iesalnieks I, von Breitenbuch P, Glockzin G, Schlitt HJ, et al. Different approaches for complete mobilization of the splenic flexure during laparoscopic rectal cancer resection. Int J Colorectal Dis. 2012; 27:1521–1529.
7. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004; 240:205–213.
8. Kim CW, Shin US, Yu CS, Kim JC. Clinicopathologic characteristics, surgical treatment and outcomes for splenic flexure colon cancer. Cancer Res Treat. 2010; 42:69–76.
9. Levien DH, Gibbons S, Begos D, Byrne DW. Survival after resection of carcinoma of the splenic flexure. Dis Colon Rectum. 1991; 34:401–403.
10. Griffiths JD. Surgical anatomy of the blood supply of the distal colon. Ann R Coll Surg Engl. 1956; 19:241–256.
11. Jamali FR, Soweid AM, Dimassi H, Bailey C, Leroy J, Marescaux J. Evaluating the degree of difficulty of laparoscopic colorectal surgery. Arch Surg. 2008; 143:762–767.
12. Akiyoshi T, Kuroyanagi H, Oya M, Ueno M, Fujimoto Y, Konishi T, et al. Factors affecting difficulty of laparoscopic surgery for left-sided colon cancer. Surg Endosc. 2010; 24:2749–2754.
13. Pisani Ceretti A, Maroni N, Sacchi M, Bona S, Angiolini MR, Bianchi P, et al. Laparoscopic colonic resection for splenic flexure cancer: our experience. BMC Gastroenterol. 2015; 15:76.
14. Fiscon V, Portale G, Migliorini G, Frigo F. Splenic flexure colon cancers: minimally invasive treatment. Updates Surg. 2015; 67:55–59.
15. Nakashima M, Akiyoshi T, Ueno M, Fukunaga Y, Nagayama S, Fujimoto Y, et al. Colon cancer in the splenic flexure: comparison of short-term outcomes of laparoscopic and open colectomy. Surg Laparosc Endosc Percutan Tech. 2011; 21:415–418.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download