Abstract
Silicone implants are widely used in aesthetic and reconstructive breast surgery. Silicone lymphadenopathy is a well-known rare complication of implant insertion. Silicone leakage from a rupture or silicone bleeding can accumulate in lymph nodes. Foreign body reactions in the affected lymph nodes may be misdiagnosed as metastasis or malignant lymphadeno pathy upon initial presentation if silicone lymphadenopathy is not considered in the initial diagnosis. We report a case of siliconoma with extensive involvement of multiple lymph nodes mimicking malignant features to emphasize that clinicians should carefully evaluate each patient's medical history and disease status during differential diagnosis.
Axillary lymphadenopathy arises from benign as well as malignant causes. Silicone implants are widely used in aesthetic and reconstructive breast surgery. The causes of axillary lymphadenopathy after implant insertion are multifactorial, and include such as granulomatous reaction, inflammation, malignancy, and silicone lymphadenopathy [1]. Silicone prostheses are thought to be biologically inert. However, in recent years, the safety of silicone implants has been examined in several studies that described ruptures, granulomatous reactions and malig nancies [2].
The relationship between silicone breast implants and malignancy has been reported in the context of lymphoma [23]. These cases of lymphoma are located mainly in the breast near the implant; however, some cases reported that axillary lymph nodes were also affected by lymphoma [1].
Silicone rupture or “bleeding” is one of causes of lymphadeno pathy after silicone implantation, because transportation of silicone particles to the regional lymph nodes leads to siliconoma. The resulting foreign body reaction may produce local swelling of the involved lymph nodes, which can be misdiagnosed as metastasis or malignant lymphadenopathy upon initial presentation [3]. If not considered in the initial differential diagnosis, siliconoma is a difficult diagnosis to arrive at. Herein, we report a case of extensive siliconoma in the axilla and neck mimicking the clinical symptoms of malignant lymph adenopathy, including fever and general weakness, in the hopes that our experience will contribute to clinical decision making.
A 35-year-old woman was referred to the breast department and presented with tender and palpable masses on her right axillary and supraclavicular area. She noticed a palpable mass on her neck 6 months prior and on her axilla 2 months before admission. She had experienced general weakness and a fever of 38℃–39℃ occurring 2 or 3 times per day during the seven days prior to her visit. Her body temperature was recorded as 38.5℃ with an ear thermometer. We performed imaging and laboratory studies to evaluate the cause of fever. There were no symptoms of upper respiratory tract infection (cough, sore throat, or runny nose) or urinary tract infection (pain during urination or low back pain). Chest X-ray revealed no abnormalities and complete blood count (CBC), liver function test (LFT) and urinary analysis were all within normal range; however, ESR, CRP, and LDH levels were increased (ESR, 25 mm/hr; CRP, 28.3 mg/L; LDH, 662 IU/L).
On physical examination, multiple movable round masses were palpated in the patient's right axilla and anterior neck. The patient reported having undergone bilateral breast augmentation with silicone implants for cosmetic purposes 11 years earlier. Initial breast ultrasound scans showed both implants to be intact, but there were multiple hyperechoic lesions with diffuse white noise posteriorly in the right axillary and supraclavicular areas (Fig. 1A). CT of the neck and chest demonstrated multiple round to oval shaped, noncontrast enhanced lymph nodes from the right axilla to the anterior neck (Fig. 1B). We performed ultrasonography-guided fine needle aspiration (FNA) and the cytologic findings were reactive lymphoid tissue without malignant cells.
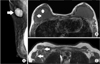
The patient underwent mammography and MRI to distinguish consequential silicone lymphadenopathy from breast implant rupture and malignancy. Mammography showed a hyper dense silicone bag implant posterior to the pectoralis major muscle in the breast and an enlarged lymph node with dense internal material in the right axilla (Fig. 2A). MRI findings included tear drop sign suggesting intracapsular rup ture within the right implant without demonstrable extracapsular droplets (Fig. 2B). MRI also demonstrated oval circumscribed lymph nodes with isosignal intensity compared to the silicone implant in her right axilla (Fig. 2C).
Despite 4 days of conservative management including first-generation cephalosporin and nonsteroidal anti-inflamma tory drugs (NSAID), the symptoms continued. This continuation of the patient's clinical symptoms, including fever and pain, concern regarding false-negative results of FNA, and inflammation of the patient's enlarged lymph nodes necessitated excision of the lymph nodes and tissue retrieval for histo pathologic examination. In addition, due to the possibility of silicone lymphadenopathy due to intracapsular rupture, we per formed a combined procedure involving excisional biopsy of the right axillary and supraclavicular lesions and removal of both implants. On macroscopic inspection, the lymph nodes were round, enlarged, and solid (Fig. 3). In the right axilla, there were multiple palpable lymph nodes at axillary levels I and II. We removed 3 enlarged lymph nodes and sent them for frozen biopsy, the findings of which were not malignant. We only removed the lymph nodes in the axilla and supraclavicular area that could be palpated on physical examination because the patient complained of the pain and cosmetic concerns. The right prosthesis was grossly ruptured with surrounding silicone leakage. Total capsulectomy was performed. Pathological examination identified lymph nodes that had been largely replaced by histiocytes containing foreign material and multinucleated giant cells, consistent with silicone lymph adenopathy (Fig. 4).
After the operation, firstgeneration cephalosporin and NSAIDs were injected for 3 days because the patient had a fever of about 38℃ and pain for 2 days. Three days after surgery, her body temperature decreased to 36℃–37℃ and the pain subsided. Closed suction drains in her right axilla and neck were removed 5 days postoerative. She was discharged 9 days later with out any wound healing problem, pain or fever.
Written informed consent was obtained from the patient for publication of this case report.
Silicone breast implant was first introduced in 1962 by Cronin and Gerow (quoted from [4]). Thereafter, breast augmentation became one of the most frequently performed cos metic surgeries. Silicone prostheses are a biologically inert sub stance that causes little local inflammation and low tissue immuno genicity. However, concerns related to the safety of sili cone implants including local inflammatory reactions, connective tissue disease, autoimmune disease and malignancy have arisen [2]. Extensive epidemiological studies do not support a causal relationship between breast implants and breast cancer or any other types of cancer, definite or aty pical connective tissue disease, adverse offspring effects, or neuro logic disease [5]. Nonetheless, although extremely rare, im plants are sometimes associated with the development of primary nonHodgkin lymphoma of the breast, primarily the anaplastic large cell lymphoma (ALCL) subtype [1]. ALCL after breast implantation was estimated by de Jong et al. [6] to have an incidence of 0.1 to 0.3 per 100,000 women with implants per year. Most cases presented with a seroma, with the capsule and breast near the implant typically affected. However, cases of axillary lymphadenopathy have also been reported [1].
Breast implant rupture, leakage or both through an intake surface can cause fibrosis and foreign body granulomatous reactions resulting in contracture or regional lymphadenopathy. Rupturefree survival of thirdgeneration implants was reported to be 98% at 5 years and 83 to 85% at 10 years [7]. In fourthgeneration implants, the overall rupture rate was reported to be 13% for subjects according to serial MRI by 10 years [8]. However, microscopic silicone droplets can migrate to body tissues even when the implant surface remains intact. Silicone particles can be transported to regional lymph nodes and granulomatous reactions may present as lymphadenopathy caused by phagocytosis of multinucleated giant cells. The largest series on breast silicone lymphadenopathy by Zambacos et al. [9] included 14 cases and the authors also reviewed 29 published articles including 175 cases; most published reports represent single case reports. However, the incidence and prevalence of this disease in the entire population of women with breast implants remains largely unknown, and no publi cations have discussed this issue directly.
Because axillary lymphadenopathies may arise from a variety of causes that are benign as well as malignant, differential diagnosis on initial presentation is essential. The current case is particularly unusual in that, to the best of our knowledge, it is the most extensively involved multiple lymphadenopathy with clinical symptoms mimicking malignant lymphadenopathy ever reported. The patient presented with palpable axilla and neck masses, multiple enlarged lymph nodes, fever and general weakness, which can be confused with B symptoms (fever, night sweats and weight loss) that may be associated with lymphoma. We sought to determine the cause of fever; however, CBC, LFT, urine analysis, and chest X-ray demonstrated normal findings. We suspected malignant lymphoma or silicone lymphadenopathy based on high LDH, enlarged lymph nodes and history of breast implantation. FNA cytology is an accurate initial investigation technique that can contribute to diagnosis of lymphadenopathy [10]. If FNA is inconclusive, or if other, more aggressive diagnoses are suspected, excisional biopsy is advisable for histological diagnosis and to exclude concomitant malignancy. In this case, FNA cytology did not indicate malignancy, and MRI suggested intracapsular leakage. Although silicone lymphadenopathy was strongly indicated, we were unable to exclude malignancy. Typically, patients with silicone lymphadenopathy present with a hard, tender palpable mass without fever because silicone prostheses are biologically inert, and there is no evidence that silicone lymphadenopathy is immunologically active. Core biopsy could facilitate differential diagnosis when masses are suspected to be malignant. In this case, the patient complained not only of pain, but also had cosmetic concerns. Therefore, we performed a combined procedure involving excisional biopsy of palpable enlarged lymph nodes, removal of both implants and capsulectomy due to the continuation of fever and tenderness.
We present this case to emphasize that silicone lymph adeno pathy should be considered in patients with a history of breast augmentation or reconstruction. In cases with extensive involve ment of the lymph nodes, a thorough examination to identify and confirm the nature of lymph node enlargement including history taking, radiology, FNA, and, if needed, excisional biopsy, should be kept in mind.
Figures and Tables
Fig. 1
Ultrasonographic and computed tomographic findings of the lymph nodes. (A) Ultrasonographic images of axillary lymph nodes. Multiple hyperechoic lesions with diffuse posterior white noise (where silicone blocks sound transmission, white arrows) were observed. (B) Coronal view of computed tomography. Round, nonenhanced enlarged lymph nodes are located in the right axilla and neck (white arrows).

Fig. 2
Mammographic and MRI of the breast and axilla. (A) Mediola teral view of mam mo graphy im ages demon strating the right breast implant and an enlarged lymph node with the dense internal material in the right axilla (white arrow). (B) Axial nonfat sup pressed, T1-weighted MRI of the breast show tear drop sign within the right implant and a hypo dense thin line in the interior of the implant indicative of intracapsular rupture (white arrows). (C) Axial nonfat suppressed, T1-weighted MRI im ages of the breast show oval cir cumscribed lymph nodes with isosignal intensity (white arrows) in the right axilla compared to the implanted silicone bag in panel B.
ACKNOWLEDGEMENTS
This research was supported by a Korea University Grant (K1609841) and the National Research Foundation of Korea (2017R1D1A1B03028103).
References
1. Gidengil CA, Predmore Z, Mattke S, van Busum K, Kim B. Breast implant-associated anaplastic large cell lymphoma: a systematic review. Plast Reconstr Surg. 2015; 135:713–720.
2. Cook PD, Osborne BM, Connor RL, Strauss JF. Follicular lymphoma adja cent to foreign body granulomatous inflammation and fibrosis surrounding silicone breast prosthesis. Am J Surg Pathol. 1995; 19:712–717.
3. Gundeslioglu AO, Hakverdi S, Erdem O, Ozen EC, Inan I, Emlik D. Axillary lipogranuloma mimicking carcinoma metastasis after silicone breast implant rupture: a case report. J Plast Reconstr Aesthet Surg. 2013; 66:e72–e75.
4. Maxwell GP, Gabriel A. The evolution of breast implants. Clin Plast Surg. 2015; 36:399–404.
5. McLaughlin JK, Lipworth L, Murphy DK, Walker PS. The safety of silicone gelfilled breast implants: a review of the epidemiologic evidence. Ann Plast Surg. 2007; 59:569–580.
6. de Jong D, Vasmel WL, de Boer JP, Verhave G, Barbe E, Casparie MK, et al. Anaplastic largecell lymphoma in women with breast implants. JAMA. 2008; 300:2030–2035.
7. Holmich LR, Friis S, Fryzek JP, Vejborg IM, Conrad C, Sletting S, et al. Incidence of silicone breast implant rupture. Arch Surg. 2003; 138:801–806.
8. Allergan Silicone Breast Implant U.S. Core Clinical Study Group. Spear SL, Murphy DK. Natrelle round silicone breast implants: Core Study results at 10 years. Plast Reconstr Surg. 2014; 133:1354–1361.
9. Zambacos GJ, Molnar C, Mandrekas AD. Silicone lymphadenopathy after breast augmentation: case reports, review of the literature, and current thoughts. Aesthetic Plast Surg. 2013; 37:278–289.
10. Buley ID. Fine needle aspiration of lymph nodes. J Clin Pathol. 1998; 51:881–885.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download