Abstract
Purpose
Duodenal stump fistula (DSF) is a serious complication after gastrectomy for gastric cancer. Although risk evaluation and management of DSF were presented by some investigators, there was no technical attempt has been made to prevent DSF during laparoscopic gastrectomy until now.
Methods
Consecutive 99 patients were enrolled from April 2014 to February 2016 in 2 institutes. All patients were performed laparoscopic gastrectomy for gastric cancer. After cutting of duodenal stump, laparoscopic reinforcement suture (LARS) commenced with continuous invagination method or interrupted method by barbed suture. Clinicopathologic features and postoperative outcomes were analyzed.
Results
Fifty-six patients had comorbidity including 5 patients with duodenal ulcer. Most patients were performed distal gastrectomy with B-II, and 10 patients total gastrectomy with Roux en Y esophagojejunostomy. Although there were 2 esophagojejunostomy leakage and 1 artificial lesser curvature leakage, DSF did not occurred at all in this study. Mean operation time was 3 hours and mean LARS procedure time was 8 minutes.
The overall incidence of duodenal stump fistula (DSF) or duodenal stump leakage is reportedly between 1.6% to 5% and is one of the most serious complications of BillrothII or Roux en Y reconstruction after gastrectomy for gastric cancer [12]. Several investigators presented their clinical experience, such as the clinical course and the pertinent management of DSF [34]. Conservative approach for DSF is the treatment of choice, eventually associated with percutaneous drainage [4]. It is possible to predict possibilities of DSF in some patients, such as patient's age, comorbidity, nutritional status impairment and technical difficulties during surgery [56]. However, surgeons make few technical attempts to prevent fistula from staple-line of duodenal stump during laparoscopic gastrectomy.
During the past 2 decades, laparoscopic gastrectomy for stage I gastric cancer has become an attractive alternative to open gastrectomy in Korea, Japan, and China [789101112]. Although the incidence of wound complication in laparoscopic gastrectomy is significantly lower in open gastrectomy, the incidence of overall complication is similar between the 2 groups [78]. However, the advantage of laparoscopic gastrectomy in major complication, such as DSF or intraabdominal bleeding remains unclear [9]. The apparent cause of DSF is an area of active investigation because of several complicated risk factors associated with DSF.
Irrespective of cause, DSF should be prevented with various methods. We have performed additional mechanical rein forcement on staple-line of duodenal stump. Herein, we introduced a new and simple surgical technique for reducing DSF during laparoscopic gastrectomy.
We retrospectively reviewed the medical records for con secutive 99 patients who underwent laparoscopic reinforcement su ture (LARS) during laparoscopic gastrectomy for gastric cancer from April 2014 to February 2016 in 2 institutes. Written informed consents were provided to all patients. The indi ca tion of laparoscopic gastrectomy for gastric cancer was less than preoperatively clinical T2N1M0, except the indication of endoscopic submucosal dissection.
We defined DSF by the presence of biliaryenteric drainage, which exists through the abdominal wall confirmed by an abdominal-pelvic CT scan or fistulography. Image studies were performed in patients who represented symptoms and signs such as severe and abrupt abdominal pain, fever, worsening leukocytosis, and clinical suspects of DSF.
We analyzed clinicopathologic features, such as age, sex, body mass index, comorbidities including duodenal ulcer, American Society of Anesthesiologists (ASA) physical status classification, tumor location, number of retrieved lymph node and TNM stage. Postoperative outcomes included LARS method, operation time, combined surgery, intraoperative blood loss, type of surgery, distance of resection margin, postoperative hospital stay, postoperative complication with Clavien-Dindo classification and mortality.
For laparoscopic gastrectomy in cases of gastric cancer, 5 trocars were used while standing at the patient's right side during the entire procedure, as described in our previous report [13]. After cutting of duodenal stump of about 1.5- to 2-cm length using linear stapler, LARS commenced from upper to lower part on staple-line of duodenal stump. Continuous suture with invagination was performed using a barbed suture (Fig. 1). In case of patient with short duodenal stump because of chronic ulcer or ectopic pancreas at duodenal bulb or cancer invasion to pylorus, 2 or 3 interrupted sutures without invagination of duodenal stump (Fig. 2) was conducted using barbed sutures.
Clinicopathologic characteristics of the patients were shown in Table 1. Mean age was 60.9 years, and 63 patients (63.6%) were male. Fiftysix patients (56.6%) had comorbidity including 5 patients with duodenal ulcers. Most patients had <2 ASA physical status classification and pathologic stage I or II cancer.
In postoperative outcomes (Table 2), continuous suture with invagination was successfully performed in 94 cases and interrupted sutures without invagination in 5 cases. The mean operation time was 179.8 minutes, and the mean time for LARS was mean 8 minutes. It took >15 minutes for this procedure in initial period. However, it took <8 minutes on average after 20 cases. There were 17 combined surgeries, including 11 cases of cholecystectomy, 2 of nephrectomy, open heart surgery, appendectomy, thyroid surgery and breast mass excision.
Postoperative complication occurred in 14 patients (14%). DSF after laparoscopic gastrectomy for gastric cancer did not occur in this study, although esophagojejunostomy leakages and leakage at artificial lesser curvature site of remnant stomach occurred in 2 and 1 case, respectively. According to Clavien-Dindo classification, 9 patients were classified as <II and 5 patients IIIa. Only 1 patient underwent surgical intervention, such as laparoscopic adhesiolysis and jejunojejunostomy, due to the postoperative ileus.
Fig. 3 presented CT finding of duodenal stump treated LARS at postoperative 6 months.
DSF is a rare complication but is associated with a high morbidity and mortality rate. The mortality rate of DSF is reported as 16% to 20% [1]. This serious complication affects not only patients and their families, but also the surgeon especially in cases with laparoscopic gastrectomy. Factors associated with DSF can be divided into patient factors and surgeon factors. Patient factors include age, comorbidity, nutritional status, existence of chronic ulcer or ectopic pancreas at duodenal bulb, cancer invasion to pylorus, gastric outlet obstruction and previous abdominal surgery, and so on [345]. Surgeon factors could be related to several surgical techniques, such as excessive vascular or pancreatic dissection around duodenal stump, direct thermal injury by ultrasonic shears and reinforcement suture [514]. In addition, DSF can also be associated with difficulties in emptying the afferent jejunal loop due to stricture of gastrojejunostomy or acute pancreatitis.
According to a multicenter randomized controlled trial (KLASS 01) for laparoscopic versus open gastrectomy for stage I gastric cancer, laparoscopic gastrectomy is safe and has a benefit of lower occurrence of wound complication [9]. However, anastomotic leakage after laparoscopic gastrectomy occurs more frequently than after open gastrectomy, without statistical significance [9]. Cozzaglio et al. [1] also reported that the laparoscopic technique increases the risk of DSF about 5 times, possibly due to learning curve of laparoscopic skills and the lack of reinforcement of the duodenal stump, which can be performed routinely in open approach.
Recently, minimally invasive surgery has been replaced by totally laparoscopic surgery from assisted laparoscopic surgery, which needs a minilaparotomy wound for specimen retrieval and reconstruction [151617]. Most experienced laparoscopic surgeons are sufficiently skilled in intracorporeal suturing for bowel reconstruction. We conducted LARS on staple-line to prevent DSF using barbed suture. Some surgeons applied fibrinsealant (Greenplasty, Green Cross Corp., Seoul, Korea) or absorbable reinforcement felt (Neoveil, Gunze Ltd., Kyoto, Japan) at staple-line of duodenal stump to prevent the DSF. However, its clinical efficacy remains unclear [2].
In laparoscopic sleeve gastrectomy for morbid obesity patients, several reinforcement techniques on staple-line, such as buttressing with specific bioabsorbable materials, oversewing, or application of sealant agents have been introduced [18192021]. In vitro, Lembert's suture reinforcement technique on stapled human stomach has less leakage rate, as compared to throughandthrough reinforcement and nonreinforced staple-line [21]. Using a systematic review with metaanalysis, the leak rate in laparoscopic sleeve gastrectomy was significantly lower using buttressing of staple-line with absorbable polymer membrane than oversewing, nonabsorbable bovine pericardial strips reinforcement, or no reinforcement [19]. However, the standard technique for prevention of leakage from staple-line in laparoscopic sleeve gastrectomy has not yet been established by welldesigned randomized prospective multicenter study.
In our study, DSF did not occur after LARS on staple-line at duodenal stump using barbed suture during laparoscopic gastrectomy for gastric cancer. Additional mechanical reinforcement after stapling of the duodenum can be helpful to prevent DSF during laparoscopic gastrectomy. Barbed suture is very useful to perform an intracorporeal suturing because laparoscopic knotting is unnecessary. Furthermore, this simple LARS procedure did not need much time and also did not increase the total operation time.
The study had some limitations including a small number of patients from 2 institutes and its retrospective nature. However, it serves as a pilot study for future multicenter randomized controlled trials on clinical significance of LARS for prevention of DSF during laparoscopic gastrectomy.
In conclusion, LARS can be performed in a relatively short operation time without any technical difficulties. LARS on staple-line of duodenal stump can be helpful to prevent DSF after laparoscopic gastrectomy for gastric cancer.
Figures and Tables
 | Fig. 1(A) Laparoscopic reinforcement suture (LARS) commence from upper to lower part on staple-line of duodenal stump using barbed suture. (B) Continuous suture with invagination is completed after 5 or 6 stitches. |
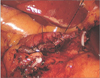 | Fig. 2In case of patient with short duodenal stump because of chronic ulcer or ectopic pancreas at duodenal bulb or cancer invasion to pylorus, 2 or 3 interrupted sutures without invagination of duodenal stump is conducted using barbed sutures. |
 | Fig. 3Duodenal stump after Laparoscopic reinforcement suture (LARS) is presented at abdominal CT scan at postoperative 6 months. Arrow indicates invaginated duodenal stump. |
ACKNOWLEDGEMENTS
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (1320270). The funding source had no role in the design of this article and will not have any role during its execution or publication.
References
1. Cozzaglio L, Giovenzana M, Biffi R, Cobianchi L, Coniglio A, Framarini M, et al. Surgical management of duodenal stump fistula after elective gastrectomy for malignancy: an Italian retrospective multicenter study. Gastric Cancer. 2016; 19:273–279.
2. Paik HJ, Lee SH, Choi CI, Kim DH, Jeon TY, Kim DH, et al. Duodenal stump fistula after gastrectomy for gastric cancer: risk factors, prevention, and management. Ann Surg Treat Res. 2016; 90:157–163.
3. Cornejo Mde L, Priego P, Ramos D, Coll M, Ballestero A, Galindo J, et al. Duodenal fistula after gastrectomy: retrospective study of 13 new cases. Rev Esp Enferm Dig. 2016; 108:20–26.
4. Aurello P, Sirimarco D, Magistri P, Petrucciani N, Berardi G, Amato S, et al. Management of duodenal stump fistula after gastrectomy for gastric cancer: systematic review. World J Gastroenterol. 2015; 21:7571–7576.
5. Orsenigo E, Bissolati M, Socci C, Chiari D, Muffatti F, Nifosi J, et al. Duodenal stump fistula after gastric surgery for malignancies: a retrospective analysis of risk factors in a single centre experience. Gastric Cancer. 2014; 17:733–744.
6. Kim KH, Kim MC, Jung GJ. Risk factors for duodenal stump leakage after gastrectomy for gastric cancer and management technique of stump leakage. Hepatogastroenterology. 2014; 61:1446–1453.
7. Kim HH, Han SU, Kim MC, Hyung WJ, Kim W, Lee HJ, et al. Longterm results of laparoscopic gastrectomy for gastric cancer: a largescale casecontrol and case-matched Korean multicenter study. J Clin Oncol. 2014; 32:627–633.
8. Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg. 2010; 251:417–420.
9. Kim W, Kim HH, Han SU, Kim MC, Hyung WJ, Ryu SW, et al. Decreased Morbidity of laparoscopic distal gastrectomy compared with open distal gastrectomy for stage I gastric cancer: shortterm outcomes from a multicenter randomized controlled trial (KLASS-01). Ann Surg. 2016; 263:28–35.
10. Etoh T, Inomata M, Shiraishi N, Kitano S. Minimally invasive approaches for gastric cancerJapanese experiences. J Surg Oncol. 2013; 107:282–288.
11. Lu W, Gao J, Yang J, Zhang Y, Lv W, Mu J, et al. Longterm clinical outcomes of laparoscopyassisted distal gastrectomy versus open distal gastrectomy for early gastric cancer: a comprehensive systematic review and meta-analysis of randomized control trials. Medicine (Baltimore). 2016; 95:e3986.
12. Kim MC, Kim W, Kim HH, Ryu SW, Ryu SY, Song KY, et al. Risk factors associated with complication following laparoscopy-assisted gastrectomy for gastric cancer: a largescale korean multicenter study. Ann Surg Oncol. 2008; 15:2692–2700.
13. Kim MC, Choi HJ, Jung GJ, Kim HH. Techniques and complications of laparoscopyassisted distal gastrectomy (LADG) for gastric cancer. Eur J Surg Oncol. 2007; 33:700–705.
14. Yakoub D, Athanasiou T, Tekkis P, Hanna GB. Laparoscopic assisted distal gastrectomy for early gastric cancer: is it an alternative to the open approach? Surg Oncol. 2009; 18:322–333.
15. Chen K, Wu D, Pan Y, Cai JQ, Yan JF, Chen DW, et al. Totally laparoscopic gastrectomy using intracorporeally stapler or handsewn anastomosis for gastric cancer: a singlecenter experience of 478 consecutive cases and outcomes. World J Surg Oncol. 2016; 14:115.
16. Kim EY, Choi HJ, Cho JB, Lee J. Totally laparoscopic total gastrectomy versus laparoscopically assisted total gastrectomy for gastric cancer. Anticancer Res. 2016; 36:1999–2003.
17. Han G, Park JY, Kim YJ. Comparison of shortterm postoperative outcomes in totally laparoscopic distal gastrectomy versus laparoscopy-assisted distal gastrectomy. J Gastric Cancer. 2014; 14:105–110.
18. Carandina S, Tabbara M, Bossi M, Valenti A, Polliand C, Genser L, et al. Staple line reinforcement during laparoscopic sleeve gastrectomy: absorbable monofilament, barbed suture, fibrin glue, or nothing? Results of a prospective randomized study. J Gastrointest Surg. 2016; 20:361–366.
19. Gagner M, Buchwald JN. Comparison of laparoscopic sleeve gastrectomy leak rates in four staple-line reinforcement options: a systematic review. Surg Obes Relat Dis. 2014; 10:713–723.
20. Al Hajj GN, Haddad J. Prevent ing staple-line leak in sleeve gastrectomy: reinforcement with bovine pericardium vs. oversewing. Obes Surg. 2013; 23:1915–1921.
21. Rogula T, Khorgami Z, Bazan M, Mamolea C, Acquafresca P, ElShazly O, et al. Comparison of reinforcement techniques using suture on staple-line in sleeve gastrectomy. Obes Surg. 2015; 25:2219–2224.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download