Abstract
Purpose
Patient, surgical, and tumor factors affect the outcome after surgical resection for hepatocellular carcinoma (HCC). The surgical factors are only modifiable by the surgeon. We reviewed our experience with curative resection for HCC in terms of surgical factors.
Methods
After analyses of the prospectively collected clinical data of 256 consecutive patients undergoing surgical resection for HCC, prognostic factors for disease-free survival (DFS) and overall survival (OS) were identified; all patients were stratified by tumor diameters > or <5 cm and their outcomes were compared.
Results
Multivariate analyses showed that microvascular invasion, estimated blood loss, blood transfusion, and the number of tumors were independent adverse prognostic factors for DFS, whereas microvascular invasion, serum alpha fetoprotein, and tumor diameter were independent adverse prognostic factors for OS. Blood transfusion had borderline significance (P = 0.076). After stratification by tumor diameter, blood transfusion was only associated with poor DFS and OS in patients with tumor diameters > 5 cm.
Hepatocellular carcinoma (HCC) is the most common primary cancer of the liver and one of the most frequent neoplasms worldwide [1]. Most cases of HCC are accompanied by liver disease induced by viral hepatitis or alcohol. It is necessary to consider both the tumor characteristics and hepatic function to determine the most appropriate treatment method, such as liver resection or liver transplantation. Hepatic resection is the treatment of choice if the patient can tolerate surgery. The majority of patients with HCC have liver cirrhosis and this makes liver resection technically demanding, and at times risky, depending on the extent of the remnant liver and functional hepatic reserve [23]. Nevertheless, the results of hepatic resection for HCC have improved markedly due to increased surgical skill and perioperative management [45].
Various prognostic factors affect the outcomes of HCC; patient factors (age, sex, laboratory findings, cirrhosis, and hepatitis virus), tumor factors (tumor diameter, number of tumors, histological grade, microvascular invasion, capsule formation, serosa invasion, and serum α-FP and proteins induced by vitamin K antagonist or absence-II [PIVKA-II]), and surgical factors (extent of resection, estimated blood loss [EBL], blood transfusion, and surgical resection margin) [6]. Of these, surgical factors, such as surgical method, extent of resection, surgical margin, intraoperative bleeding, and blood transfusion are modifiable only by the surgeon; patients and tumor factors cannot be altered. Therefore, the purpose of this study was to review our experience with curative resection for HCC in terms of surgical factors.
We prospectively collected the clinical data of 271 consecutive patients who underwent surgical resection for HCC from January 2010 to December 2014 by 2 surgeons (DGK, YKY) at Seoul St. Mary Hospital. In total, 256 consecutive patients were enrolled after applying the following exclusion criteria: palliative resection such as tumor-involved surgical margin (n = 10), incomplete removal of tumor/thrombus from the portal vein or bile duct (n = 1), HCC-cholangiocarcinoma mixed tumor (n = 3), and perioperative mortality within 30 days of surgery (n = 1). The clinical data were reviewed after approval by the Institutional Review Board of Seoul St. Mary Hospital (KC16RISI1021). Patients were followed until March 2016.
Preoperative liver biochemistry tests were performed. Child-Pugh score and model for end-stage liver disease (MELD) score were also calculated. The indocyanine green (ICG) test was performed to evaluate residual hepatic function. Serum α-FP and PIVKA-II were assessed as tumor markers. All patients were staged before surgery using abdominal and chest CT, MRI, and 2-18F-fluoro-2-deoxy-d-glucose positron emission tomography (FDG-PET). If extrahepatic metastases or tumor thrombi were identified in the main portal vein, the patients were excluded from curative resection. Patients with a large volume of ascites or hyperbilirubinemia, as well as those who corresponded to Child class C, were also excluded; however, partial hepatectomy was performed in Child class B patients. The safe limit for the ICG retention value on the ICG test was <15% at 15 min for major hepatectomy. We performed a partial hepatectomy for patients with an ICG retention value >15%. Liver resection was performed in accordance with the Couinaud segmentation to implement hepatic segmentectomy or combined resection for adjacent liver segments (anatomical resection), or partial hepatectomy containing tumor (nonanatomical resection). Major hepatectomy was defined as resection of 2 hepatic sections/3 segments or more, and minor hepatectomy was resection of 1 section or less. Laparoscopic hepatectomy was performed in selected patients. During the operation, we do not use the Pringle maneuver routinely. The largest tumor diameter was chosen in cases of multiple HCC. EBL was collected from the anesthetic record. Blood transfusion was defined as a transfusion of red blood cells, whereas transfusions of other blood products, such as fresh-frozen plasma, platelets or albumin, were not considered. Curative resection was defined as complete removal of the tumor with a clear microscopic margin. Tumor stages were based on the 7th edition of the American Joint Committee on Cancer TNM staging system.
Each patient was managed with a standardized treatment protocol. A follow-up abdominal CT scan was performed on day 7 after surgery to evaluate intra-abdominal status. After discharge, we assessed tumor markers, such as α-FP and PIVKA-II, in the outpatient clinic at intervals of 4 months for the first year after surgery. During the second year after surgery, tumor markers were evaluated at intervals of 3 months, and CT was performed every 6 months for the next year and then annually thereafter. If recurrence was suspected or other abnormal findings were noted, liver MRI and PET-CT were performed.
Continuous data are provided as medians with ranges. The Mann-Whitney U-test or Student t-test was used to analyze the continuous data, and the chi square test or Fisher exact test was employed to assess categorical data. The primary and second endpoints were overall survival (OS) and disease-free survival (DFS), respectively. Survival curves were generated using the Kaplan-Meier method, and the log-rank test was used to compare survival. Only variables with P-values < 0.1 in the univariate analysis were included in the multivariate analysis, which was performed using Cox proportional hazards regression model. A P-value < 0.05 was considered significant. All statistical analyses were performed using IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA).
The preoperative features of the 256 patients according to tumor recurrence are described in Table 1. Of the 256 patients, 224 (88%) were diagnosed with HCC during regular health screening, routine follow-up for liver disease, or work-up for another disease. Sixty-seven patients underwent preoperative treatment such as transarterial chemoembolization, percutaneous ethanol injection, radiotherapy or a combination of them. Most patients were in Child class A (n = 242, 95%), and the others were in Child class B.
The extent of hepatic resection included extended right hemihepatectomy (n = 3, 1%), hemihepatectomy (n = 105, 41%), sectionectomy (n = 40, 16%), and partial hepatectomy (n = 108, 42%). Anatomical resection including caudate lobectomy was performed in 162 patients (63%) and laparoscopic liver resection was performed in 47 patients (18%). A laparoscopic approach was used for the hemihepatectomy (n = 2), sectionectomy (n = 10), and partial hepatectomy (n = 35). Of the 256 patients, 67 (26%) had tumors > 5 cm and 37 (15%) had multiple HCCs. Microvascular and serosal invasion was identified in 62 (24%) and 69 (27%) patients, respectively. Continuous variables, such as MELD score, serum AFP and PIVKA-II levels, and EBL differed between patients with and without recurrence, with the exception of age (P < 0.05). Number and diameter of the tumor, presence of microvascular invasion, EBL, and transfusion were correlated with tumor recurrence (P < 0.05) (Table 1).
During a median follow-up duration of 31.2 months (range, 1–75 months), tumor recurrence occurred in 119 patients (46%), and 53 patients (21%) died. The most common site of tumor recurrence was the remnant liver (n = 110, 92%), lung (n = 16, 13%), bone (n = 7, 6%), peritoneal seeding (n = 4, 3%), and lymph node (n = 3, 2%). DFS and OS of all patients at 1, 3, and 5 years were 69%, 52%, and 38% and 90%, 78%, and 74%, respectively (Fig. 1).
In the univariate analysis for DFS, MELD score, α-FP, number and diameter of tumors, microvascular and serosal invasion, major/minor resection, EBL, and transfusion were significant, whereas in the univariate analysis for OS, α-FP, tumor diameter, microvascular and serosal invasion, major/minor resection transfusion were significant (P < 0.05). Multivariate analyses showed that microvascular invasion, blood transfusion, EBL, and the number of tumors were independent adverse prognostic factors for DFS, whereas microvascular invasion and α-FP were independent adverse prognostic factors for OS (Table 2). Blood transfusion and tumor diameter had borderline significance in the multivariate analysis for OS (P = 0.088, P = 0.094, respectively).
We subclassified patients according to tumor diameter of 5 cm and compared the outcomes between groups for surgeon-correctable factors, such as detailed operation method (major/minor resection, anatomical/nonanatomical, and open/laparoscopic approach), tumor margin status, EBL and transfusion. Operation method had no effect on DFS or OS in the univariate analysis, regardless of tumor diameter (data not shown). The extent of the tumor margin did not contribute to survival in either subgroup. In univariate analyses for DFS and OS, EBL and blood transfusion were associated with poor outcomes in patients with tumor diameters > 5 cm (Fig. 2), and then we performed multivariate analysis for DFS and OS in that group (Table 3). Blood transfusion was only the independent risk factor for DFS and had borderline significance in multivariate analyses for OS.
Surgical resection is a curative treatment modality for HCC; however, the major obstacle to improved survival and prognosis in patients with HCC is the high recurrence rate after surgery. The life expectancy of patients with HCC is hard to predict, making it difficult to determine the patient's prognosis. Many factors, such as the patient's general condition (age, sex, coexisting hepatitis, liver function, and α-FP level), tumor status (tumor diameter, number, capsule formation, vessel invasion, and differentiation) are proven significant prognostic factors [6]. Operation-related factors, such as anatomical/nonanatomical resection, open/laparoscopic resection, extent of resection, surgical margin, intraoperative bleeding, and blood transfusion, are only modifiable by the surgeon. We attempted to clarify the risk factors for HCC recurrence and patient survival after hepatic resection in terms of surgical factors.
Microvascular invasion and number of tumors were independent adverse prognostic factors for DFS, while microvascular invasion and α-FP were independent adverse prognostic factors for OS. Tumor factors have been mostly proven to be independent prognostic factors for the DFS and OS of patients. It is reasonable to consider that multiple tumors, microvascular invasion, tumor size, and serum α-FP index the aggressiveness of the tumor, consequently affecting surgical results. However, a few studies have reported conflicting results [78].
Notably, anatomic resection did not significantly affect tumor recurrence or the survival of patients in the present study. Although some authors have reported that anatomic resection achieves better DFS and OS than nonanatomic resection [91011], other reports are consistent with our results [121314]. Authors insisting superiority of anatomic resection hypothesized that systematic removal of a hepatic segment confined by tumor-bearing portal tributaries effectively eradicates intrahepatic HCC metastases because of the high likelihood of cancer cells from HCC spreading through the portal venous system. However, spreading through the portal venous system cannot be completely blocked by anatomic resection, as mobilizing the liver for a good surgical view through the laparotomy site may squeeze the tumor and dislodge tumor cells into the portal venous or hepatic venous tributaries.
A resection margin of at least 1 cm is commonly used by many surgeons; however, the role of the resection margin in contributing to the long-term survival of patients remains controversial. Although some authors advocate a definite resection margin > 1 cm and reported that this could definitely prolong OS of patients [151617], others found no significant effect of the surgical margin on tumor recurrence and survival [111418], consistent with our study. As many patients with HCC have coexisting hepatitis or cirrhosis, hepatic function reserve is frequently suboptimal, which is an obstacle for major and/or anatomic resection. In addition, a cirrhotic liver due to chronic hepatitis has a likelihood of multicentric carcinogenesis. In our study, 46% of all patients experienced recurrence after curative resection and the majority of these recurrences were at multicentric locations away from the resection margin, as previous studies [141920]. A detailed, balanced and deliberate decision is necessary because increasing the tumor-free margin will lead to resecting more nontumorous liver.
Considerable interest has arisen on the effect of blood transfusions on HCC recurrence after hepatectomy, with respect to improvement of the postoperative prognosis. A blood transfusion may have a deleterious effect on recurrence and the survival of patients due to immunosuppression, which is not fully understood, several studies have suggested that blood transfusions suppress host immunity via a toxic T-cell function, increased numbers of suppressor T cells and decreased function of macrophages and monocytes [212223]. Several studies have reported that HCC frequently recurs after a perioperative blood transfusion [242526]. In our study, EBL and blood transfusion were independent factors determining the recurrence of HCC. In addition, blood transfusion had borderline significance as a prognostic factor for OS. Furthermore, in our subgroup analyses according to a tumor diameter of 5 cm, blood transfusion had an adverse effect on DFS and OS, mainly in patients with tumor diameters > 5 cm. Thus, the primary aim of the surgeon during hepatectomy should be to achieve the least bleeding and blood transfusion, particularly for patients with large tumors through hepatic inflow control (Pringle maneuver). However, if using the Pringle maneuver, there might be ischemia-reperfusion injury. There has been long debated for the potential of liver remnant ischemia-reperfusion injury and its resultant impact on tumor progression [27282930]. Most of studies arguing that ischemia-reperfusion injury may promote progression of HCC were experimental for microenvironmental condition such as disrupting hepatic microvasculature, antiapoptosis induced by proinflammatory cytokine. As a clinical surgeon, it is more reasonable to practice as clinical studies rather than to follow experimental studies on condition that the hypothesis would not be proven.
In conclusion, the surgical technique is as important as preoperative liver function and tumor status in terms of tumor recurrence, which leads to poor patient survival. The ability of the surgeon to minimize bleeding and blood transfusion improves the outcomes of patients, particularly in cases with a large tumor diameter. After carefully selecting patients through liver function screening, a meticulous surgical technique is highly important for an improved hepatectomy outcome for HCC.
Figures and Tables
 | Fig. 1Disease-free survival (A) and overall survival (B) of all patients at 1, 3, and 5 years were 69%, 52%, 38% and 90%, 78%, 74%, respectively. |
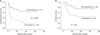 | Fig. 2Disease-free survival (A) and overall survival (B), according to transfusion in patients with tumor > 5 cm. |
References
1. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011; 365:1118–1127.
2. European Association For The Study Of The Liver. European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012; 56:908–943.
3. Korean Liver Cancer Study Group (KLCSG). National Cancer Center, Korea (NCC). 2014 Korean Liver Cancer Study Group-National Cancer Center Korea Practice Guideline for the Management of Hepatocellular Carcinoma. Korean J Radiol. 2015; 16:465–522.
4. Fan ST, Mau Lo C, Poon RT, Yeung C, Leung Liu C, Yuen WK, et al. Continuous improvement of survival outcomes of resection of hepatocellular carcinoma: a 20-year experience. Ann Surg. 2011; 253:745–758.
5. Han DH, Choi GH, Park JY, Ahn SH, Kim KS, Choi JS, et al. Lesson from 610 liver resections of hepatocellular carcinoma in a single center over 10 years. World J Surg Oncol. 2014; 12:192.
6. Qin LX, Tang ZY. The prognostic significance of clinical and pathological features in hepatocellular carcinoma. World J Gastroenterol. 2002; 8:193–199.
7. Fan ST, Ng IO, Poon RT, Lo CM, Liu CL, Wong J. Hepatectomy for hepatocellular carcinoma: the surgeon's role in long-term survival. Arch Surg. 1999; 134:1124–1130.
8. Sim HG, Ooi LL. Results of resections for hepatocellular carcinoma in a new hepatobiliary unit. ANZ J Surg. 2003; 73:8–13.
9. Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, Minagawa M, et al. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg. 2005; 242:252–259.
10. Hwang S, Lee YJ, Kim KH, Ahn CS, Moon DB, Ha TY, et al. The impact of tumor size on long-term survival outcomes after resection of solitary hepatocellular carcinoma: single-institution experience with 2558 patients. J Gastrointest Surg. 2015; 19:1281–1290.
11. Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003; 38:200–207.
12. Park JH, Koh KC, Choi MS, Lee JH, Yoo BC, Paik SW, et al. Analysis of risk factors associated with early multinodular recurrences after hepatic resection for hepatocellular carcinoma. Am J Surg. 2006; 192:29–33.
13. Tanaka K, Shimada H, Matsumoto C, Matsuo K, Nagano Y, Endo I, et al. Anatomic versus limited nonanatomic resection for solitary hepatocellular carcinoma. Surgery. 2008; 143:607–615.
14. Zhang XF, Meng B, Qi X, Yu L, Liu C, Liu XM, et al. Prognostic factors after liver resection for hepatocellular carcinoma with hepatitis B virus-related cirrhosis: surgeon’s role in survival. Eur J Surg Oncol. 2009; 35:622–628.
15. Predictive factors for long term prognosis after partial hepatectomy for patients with hepatocellular carcinoma in Japan. The Liver Cancer Study Group of Japan. Cancer. 1994; 74:2772–2780.
16. Ikai I, Arii S, Kojiro M, Ichida T, Makuuchi M, Matsuyama Y, et al. Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer. 2004; 101:796–802.
17. Lise M, Bacchetti S, Da Pian P, Nitti D, Pilati PL, Pigato P. Prognostic factors affecting long term outcome after liver resection for hepatocellular carcinoma: results in a series of 100 Italian patients. Cancer. 1998; 82:1028–1036.
18. Poon RT, Fan ST, Ng IO, Wong J. Significance of resection margin in hepatectomy for hepatocellular carcinoma: a critical reappraisal. Ann Surg. 2000; 231:544–551.
19. Adachi E, Maeda T, Matsumata T, Shirabe K, Kinukawa N, Sugimachi K, et al. Risk factors for intrahepatic recurrence in human small hepatocellular carcinoma. Gastroenterology. 1995; 108:768–775.
20. Belghiti J, Panis Y, Farges O, Benhamou JP, Fekete F. Intrahepatic recurrence after resection of hepatocellular carcinoma complicating cirrhosis. Ann Surg. 1991; 214:114–117.
21. Blumberg N, Heal JM. Effects of transfusion on immune function. Cancer recurrence and infection. Arch Pathol Lab Med. 1994; 118:371–379.
22. Gascon P, Zoumbos NC, Young NS. Immunologic abnormalities in patients receiving multiple blood transfusions. Ann Intern Med. 1984; 100:173–177.
23. Kaplan J, Sarnaik S, Gitlin J, Lusher J. Diminished helper/suppressor lymphocyte ratios and natural killer activity in recipients of repeated blood transfusions. Blood. 1984; 64:308–310.
24. Asahara T, Katayama K, Itamoto T, Yano M, Hino H, Okamoto Y, et al. Perioperative blood transfusion as a prognostic indicator in patients with hepatocellular carcinoma. World J Surg. 1999; 23:676–680.
25. Hanazaki K, Kajikawa S, Shimozawa N, Matsushita A, Machida T, Shimada K, et al. Perioperative blood transfusion and survival following curative hepatic resection for hepatocellular carcinoma. Hepatogastroenterology. 2005; 52:524–529.
26. Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000; 232:10–24.
27. Arii S, Teramoto K, Kawamura T. Current progress in the understanding of and therapeutic strategies for ischemia and reperfusion injury of the liver. J Hepatobiliary Pancreat Surg. 2003; 10:189–194.
28. Man K, Ng KT, Lo CM, Ho JW, Sun BS, Sun CK, et al. Ischemia-reperfusion of small liver remnant promotes liver tumor growth and metastases: activation of cell invasion and migration pathways. Liver Transpl. 2007; 13:1669–1677.
29. Ozaki M, Todo S. Surgical stress and tumor behavior: impact of ischemia-reperfusion and hepatic resection on tumor progression. Liver Transpl. 2007; 13:1623–1626.
30. Shimoda M, Iwasaki Y, Sawada T, Kubota K. Protective effect of ischemic preconditioning against liver injury after major hepatectomy using the intermittent pringle maneuver in swine. Pathobiology. 2007; 74:42–49.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download