Abstract
Purpose
To investigate the effects of a sustained-released mixture of vascular endothelial growth factor 165 (VEGF165) and fibrin glue (FG) local administration on postoperative rabbit ileal anastomoses.
Methods
One hundred twenty-eight male and female New Zealand white rabbits underwent intraperitoneal infection subsequent ileal anastomosis surgery were divided randomly into 4 groups, including 32 animals in each, applied with saline solution, FG, rhVEGF165 and a mixture of rhVEGF165 with FG (VEGF + FG) on the anastomoses, respectively. The incidences of anastomotic leakage were observed. Histopathological examination for inflammatory infiltration, fibroblast proliferation, and capillary vascular proliferation were performed. Then, bursting pressure and hydroxyproline concentrations were assessed in anastomoses sits on postoperative days 3, 5, 7, and 14.
Results
Rabbits in VEGF + FG group had the lowest incidence of leakage (P < 0.05). Histological evaluations revealed that granulation tissue was formed on days 5 after anastomosis; fibroblast proliferation and capillary vascular proliferation were significantly increased on days 7 and 14 in VEGF + FG group. Furthermore, there was a statistically significant difference in the mean bursting pressures between VEGF + FG group and other groups on days 7 and 14 (P < 0.05), and rabbits in VEGF + FG group exhibited a higher concentration than VEGF group (P < 0.05) and FG group (P < 0.05) on day 14.
Wound healing of intestinal anastomoses still remains an interesting topic focused by many researchers. Anastomotic leak is a dreaded complication in intestinal surgery with the incidence ranging from 5% to 20% [1], and even anastomotic leakage rate is higher especially under the conditions of intraperitoneal infection, intestinal necrosis, and intestinal wall inflammatory edema. Complication arising from the failure of anastomotic healing is a major leading to increased mortality rate, repeated surgical procedures, prolonged hospitalization time, and impaired quality of life. Therefore, it is important to improve the success rate of intestinal anastomotic healing in inflammatory conditions. Many factors have been found to be related to anastomotic failure, and the primary contributor to anastomotic failure is tissue ischemia. In addition, the formation of anastomosis leads to a certain degree of devascularization which influences the anastomotic healing. Therefore, angiogenesis is considered to be one of the key factors in anastomotic healing [2].
Vascular endothelial growth factor (VEGF) has been shown to be the fundamental regulator that stimulates the formation of new blood vessels [3], playing an important role in modulating of ischemic wound healing. VEGF165, an abundant and bioactive form of the four known human isomers, is efficient for therapeutic angiogenesis in an interventional occlusion pig model. In addition, application of exogenous VEGF has been shown to improve wound healing in multiple myocutaneous flap models [4]. However, rapid intracoronary administration of rhVEGF165 solution can induce severe hypotensive shock [5]. While local injection of liquid formulations containing rhVEGF165 may induce malformed vessels during embryonic neovascularization [6]. In addition, neovascularization and vascular perfusion can be induced by controlled-release of liquid rhVEGF165 in a rabbit model of partial limb ischemia. Therefore, it is important to explore an ideal method of application of VEGF in anastomotic healing.
Fibrin glue (FG) is used by many surgeons for the promotion of local tissue growth and repair. At present, it is reported that the application of FG releasing VEGF has good effect in skin flap and ischemic diseases [789]. In addition, FG has also been utilized as a vehicle for drug delivery to treat variety diseases [1011]. But the local application of FG has not been found in the intestinal anastomosis model. Thus, we performed FG mixed with rhVEGF165 on a rabbit model of ileal anastomosis with abdominal infection to describe the actions of long-term locally delivered rhVEGF165 on anastomotic leakage healing.
One hundred twenty-eight male and female New Zealand white rabbits weighing from 2 to 3 kg were obtained from the Animal experiment center, Dalian medical university. Animals were fed with ad libitum access to the laboratory chow, except for the first 12 hours after the operation. Then, the rabbits were divided randomly into 4 groups, including 32 animals in each. All animal experiments were performed in accordance with the Guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
Bottle A (containing the FG) and bottle B (including catalyst) were dissolved by dissolving solution respectively. 200-ng rhVEGF165 was extracted and was injected into the catalyst bottle. After gently shake up and down, solutions from bottles A and B were extracted by the syringe, and then the joint is connected with the propeller and spray nozzle.
Anesthesia was achieved with an intramuscular injection of Ketamine (50 mg/kg). The abdomen was shaved and disinfected the area with polyvidone-iodine solution (Lierkang Disinfection Technology Co., Ltd., Shandong, China), and a 5- to 6-cm longitudinal skin midline incision was made with a scalpel. After median laparotomy, the cecum was identified, and then, the cecum was located by using blunt anatomical forceps to isolate and exteriorized it. A diameter of 0.5 cm hole was incised (5 cm away from the end of cecum), and the cecum was sutured at the designated position (1 cm in distance from 2 sides of the hole) and hung in the right side of the abdominal wall. Then, the peritoneum, fasciae, abdominal musculature and skin were sutured by 4-0 proline ligatures. To prevent shock, animals were performed with the subcutaneous injection of warm 37℃ saline solution (50 mL/kg). Animals were returned to cages after resuscitation where access to water and food is available. All the procedures of cecal ligation and puncture were referred to the modification protocol published on 11 December 2008 [12].
Twelve hours after establishment of abdominal infection model, anesthesia was performed with an intravenous injection of sodium pentobarbital (15 mg/kg). After undergoing laparotomy through original incision, the incised hole on cecum was stitched up. Then, 2 different segments of the ileum (10 cm away from the end of cecum) were transected without disturbing the blood circulation, and a layer of discontinuous suture was performed for the end of ileum and ileum by using 0 silk threads with a total of 14 to 16 needles. Control group: 2-mL saline was applied on the surface of anastomoses. FG group: FG was applied on the surface of intestinal anastomoses, 0.2 cm in thickness and 2 cm in width. VEGF group: 100-ng rhVEGF165 (GenScript Biotechnology Corp., Nanjing, China) was performed circularly for realization of the intestinal anastomoses. VEGF + FG group: rhVEGF165-fibrin glue (containing 100-ng rhVEGF165) with 0.2 cm in thickness and 2 cm in width was circularly treated on the surface of intestinal anastomoses. The right side of the greater omentum was simultaneously removed in order to exclude the influence of omentum adhesion on healing of anastomoses. Each rabbit intestinal anastomosis operation time was 30–50 minutes. One senior surgeon, blinded to group assignments, operated in each segment of anastomosis.
Intestinal contents and abscess around the anastomoses were considered as anastomotic leakage. The healing of anastomosis in abdomen and the incidence of leakage were observed.
The strength of each anastomosis was assessed by measuring bursting pressure on days 3, 5, 7, and 14 after administration. The bursting pressure was measured using a specifically designed apparatus without removing the adhesions. Briefly, both ends of the resected bowel segments containing the anastomotic region were ligated. The proximal end was ligated to a plastic nipple connecting to an infusion pump with methylene blue solution mixed with physiological saline at a flow rate of 2 mL/min, and the distal end was ligated to a pressure measurement transducer of the monitor. In case of any anastomotic leak, the peak pressures recorded before bursting or leakage was recorded on the monitor as the end point in millimeter mercury (mmHg) units.
Longitudinal incision of the bowel was made and a 10-mm segment around the anastomoses was collected for further analysis. The anastomotic tissues were divided into 2 segments, with one fixed in 10% formaldehyde for hematoxylin and eosin (H&E) staining and the other for Hydroxyproline concentration measurement. Specimens for H&E staining were dehydrated, embedded in paraffin, cut into 5-mm sections and stained using H&E. Mucosal anastomotic healing, inflammatory cell infiltration, granulations growth, fibroblast proliferation and capillaries proliferation were graded semiquantitatively as follows: 0, none; 1, mild; 2, moderate; 3, severe. The scores of staining were evaluated by 2 independent pathologists without knowledge of the clinicopathological features, and any difference in interpretation was resolved by consensus.
The concentration of hydroxyproline in the anastomotic tissue (wound plus 1 mm of tissue on each side) was detected using Hydroxyproline assay kit (Uscn Life Science Inc., Wuhan, China) by spectrophotometric determination at 550 nm on days 3, 5, 7, and 14 after administrations.
Statistical analysis was performed by SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA). Data was collected from study group and postoperative day. Data are presented as mean values ± standard deviation. Differences between means were assessed using the paired t-test. The chi-square test was used to analyze the incidence of anastomotic leakage. The level of statistical significance was set at 0.05.
In this study, 7 animals died during the experimental process, including 1 in the control group, 2 in the FG group, 2 in the VEGF group, and 2 in the VEGF + FG group. Intestinal contents or abscess around the anastomoses were considered as anastomotic leakage. The incidence of anastomotic leakage in VEGF + FG group was lowest than control group, FG group, and VEGF group (P < 0.05) (Table 1).
Histological evaluations provided visual evidence for the injury of anastomosis and the healing efficacy of FG, VEGF and VEGF + FG. As demonstrated in Fig. 1A, H&E staining of controls showed a large amount of pus attached to the surface of the intestinal mucosa and inflammatory cell infiltration on days 3, few granulation tissue growths on days 5 and 7, and little of fibroblast and capillary vascular proliferation on days 14. Fewer Inflammatory cell infiltration on days 3, obvious growth of granulation tissue on days 5, and small amounts of fibroblast proliferation arranged irregularly on days 14 were observed in FG group. Tissue edema was found in VEGF group and a small amount of fibroblast on days 14 were also observed. In VEGF + FG group, obvious granulation tissue was formed on days 5 after anastomosis; on days 7 and 14, fibroblast proliferation and capillary vascular proliferation were significantly increased than FG group.
In all groups, bursting pressures were further measured at the site of anastomosis and the data were shown as means ± standard deviation. As indicated in Fig. 2, there was a statistically significant difference in the mean bursting pressures between VEGF + FG group and other groups on days 7 and 14 (P < 0.05). However, the mean bursting pressure was not obviously elevated in VEGF + FG group on days 3 and 5, compared to others.
We measured hydroxyproline concentrations in the colonic anastomoses on day 3, 5, 7, 14 after administration. As shown in Fig. 3, on days 5, 7, 14, the levels of hydroxyproline concentrations in VEGF + FG administration group was significantly higher than that in control group (*P < 0.05 or **P < 0.01). In addition, the VEGF + FG group exhibited a higher concentration than VEGF group (ΔP < 0.05) and FG group (#P < 0.05) on day 14.
Infection of anastomose has an extremely serious adverse effect on the healing of colonic anastomoses. The tendency of colonic anastomosis to disrupt was extremely high if infection was encountered at surgery [13]. Thus, it is necessary to reduce the incidence of infection at intestinal surgery. In the present study, we established an abdominal infection rabbit model to describe the FG loaded rhVEGF165 on ileal anastomosis healing. The results indicated that the local application of FG plus VEGF significantly increased bursting pressures and hydroxyproline concentrations compared to the control, FG, and VEGF groups on day 14 after administration. Morphological observation showed the lowest incidence of anastomotic leakage in VEGF + FG group compared with other groups. In VEGF + FG group, granulation tissue growth, fibroblast proliferation and capillary vascular proliferation were significantly observed. The findings suggested that VEGF plus VEGF administration accelerated wound healing in anastomosis and strengthened the anastomosis.
Our findings were consistent with previous studies. Mittermayr et al. [14] demonstrated that rhVEGF165 was released from fibrin sealant at clinically appropriate times and could increase the viability of tissue flaps in vivo. In a rabbit ischemic hind limb model, VEGF-loaded nanoparticle–fibrin gel complex markedly increased in the recovered calf blood pressure and was proved to be effective in revascularization potential [15]. Yang et al. [16] conducted a preliminary study in rats to investigate the prefabrication of vascularized scaffold with the desired shape and microarchitecture combined with rhVEGF165 to mimic autogenous vascularized bone graft. They found that the sustained release of rhVEGF165 lasted 14 days in the absence of plasmin and 12 days in the presence of plasmin in rhVEGF165-fibrin sealant scaffold group, and the scaffolds with the desired shape and microarchitecture combined with rhVEGF165 could shorten the time needed for the construction of prefabricated vascularized grafts and accelerate the maturation of the vessels.
FG is widely used in the clinics as a hemostatic agent or a sealant to for decades. More recently, FG has been investigated as a local delivery of factors or drugs. Here many of those applications are discussed in the realm of analgesia, chemotherapy, infection, gene delivery and regenerative medicine [171819]. In a rat cranial model, scaffolds comprising FG loaded with 5 µg of embryonically derived heparin sulfate were applied for the enhancement of bone repair [20]. Kim et al. [21] revealed that sustained perivascular delivery of paclitaxel with FG effectively inhibited neointimal hyperplasia in rat jugular vein after open cutdown. This study provides sustained release methods for autologous bone graft and growth factor-based therapies. Wong et al. [22] performed angiogenic exogenous growth factors interacted with FG scaffold to assess the release of VEGF165, and VEGF121. This study demonstrated that this material has the biomimetic capabilities relevant to tissue engineering and other therapies, impinged upon the natural interactions between FG and growth factors. In this study, we found that fibroblast proliferation and capillary vascular proliferation were significantly increased on days 7 and 14, meanwhile bursting pressures and hydroxyproline concentrations were significantly higher on days 14 in VEGF + FG group, speculating that the delivery system composed by FG and rhVEGF165 sustained release rhVEGF165 in 2 weeks. In addition, FG with rhVEGF165 performed better than FG or VEGF alone. This result showed that application of FG loaded VEGF could obviously prolong the effect of drugs and enhanced intestinal tissue healing.
Although FG has been proved to prevent anastomotic leakage in clinical, controversial study has reported that there were postoperative complications [23]. Since it is difficult to perform local injection of VEGF into the entire colonic circumference in clinical practice, and local injection liquid solutions containing VEGF may cause adverse effects [52425], including vessels malformation, vascular perfusion or transient angiogenesis, it is urgent to explore an ideal method for wound healing. Reports have already revealed that administration of a formulation of VEGF mixed with FG into the operative site is effective for wound healing [26]. As a carrier, a mixture FG and VEGF may protect VEGF from degradation in liquid solution, and VEGF will be gradually absorbed with FG and sustained release. Our findings revealed that postoperative bursting pressures and hydroxyproline concentrations increased gradually, and a significant increasing was observed from days 7 in VEGF + FG group. Likewise, the histopathological examination was also complied with this result. Additionally, in the present study, adverse signs such as anastomotic bleeding and transient angiogenesis from VEGF + FG group were not observed. FG mixed with VEGF may avoid the degradation of VEGF in liquid solution, and reduce and even eliminate the adverse reactions in transfusion as well as the reaction of compatibility of medicine in infusing pipe, proving that a mixture formulation of FG and VEGF was an ideal method for wound healing.
Obviously, in vivo pharmacokinetic studies should be further analyzed to confirm the result of sustaining release. In addition, further evaluation in relevant preclinical models would be important to support the potential of such mixtures for the clinical treatment of ileal anastomotic leakage. Even different types of glues are still needed to elucidate the most suitable method for ileal anastomoses and are fundamental before its clinical application. Finally, further study should be investigated to identify the effect of VEGT + FG on anastomosis without infection in the future.
Overall, the data presented in this report supports that a sustained, local delivery of FG with rhVEGF165 can provide a method to reduce inflammation, stimulate neovascularization, and enhance the wound healing of ileal anastomoses in an ileal anastomosis with abdominal infection rabbit model. These findings may provide a theoretical basis for clinical prevention of intestinal anastomosis.
Figures and Tables
Fig. 1
Histological appearance of the ileal anastomosis under H&E staining in different groups. (A) Control group (×200), (B) FG group (×200), (C) VEGF group (×200), and (D) VEGF + FG group (×200). VEGF, vascular endothelial growth factor; FG, fibrin glue.
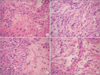
Fig. 2
Bursting pressures were detected in the anastomosis on postoperative days 3, 5, 7, and 14. Pressures were shown in millimeters mercury column (mean ± standard deviation). *P < 0.05, compared with control group. #P < 0.05, compared with FG group. ΔP < 0.05, compared with VEGF group. VEGF, vascular endothelial growth factor; FG, fibrin glue.

Fig. 3
Hydroxyproline concentrations were assessed in the anastomosis on postoperative days 3, 5, 7, and 14. Concentrations were shown in millimeters mercury column (mean ± standard deviation). *P < 0.05, **P < 0.01, compared with control group. #P < 0.05, compared with FG group. ΔP < 0.05, compared with VEGF group. VEGF, vascular endothelial growth factor; FG, fibrin glue.

References
1. Matthiessen P, Hallböök O, Rutegård J, Simert G, Sjödahl R. Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial. Ann Surg. 2007; 246:207–214.
2. Thompson SK, Chang EY, Jobe BA. Clinical review: healing in gastrointestinal anastomoses, part I. Microsurgery. 2006; 26:131–136.
3. Shibuya M. Structure and function of VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct Funct. 2001; 26:25–35.
4. Seify H, Bilkay U, Jones G. Improvement of TRAM flap viability using human VEGF-induced angiogenesis: a comparative study of delay techniques. Plast Reconstr Surg. 2003; 112:1032–1039.
5. Henry TD, Rocha-Singh K, Isner JM, Kereiakes DJ, Giordano FJ, Simons M, et al. Intracoronary administration of recombinant human vascular endothelial growth factor to patients with coronary artery disease. Am Heart J. 2001; 142:872–880.
6. Drake CJ, Little CD. Exogenous vascular endothelial growth factor induces malformed and hyperfused vessels during embryonic neovascularization. Proc Natl Acad Sci U S A. 1995; 92:7657–7661.
7. Zhang L, Zhang L, Lan X, Xu M, Mao Z, Lv H, et al. Improvement in angiogenesis and osteogenesis with modified cannulated screws combined with VEGF/PLGA/fibrin glue in femoral neck fractures. J Mater Sci Mater Med. 2014; 25:1165–1172.
8. Shireman PK, Greisler HP. Mitogenicity and release of vascular endothelial growth factor with and without heparin from fibrin glue. J Vasc Surg. 2000; 31:936–943.
9. Wu X, Ren J, Li J. Fibrin glue as the cell-delivery vehicle for mesenchymal stromal cells in regenerative medicine. Cytotherapy. 2012; 14:555–562.
10. Ozaki S, Saito A, Nakaminami H, Ono M, Noguchi N, Motomura N. Comprehensive evaluation of fibrin glue as a local drug-delivery system-efficacy and safety of sustained release of vancomycin by fibrin glue against local methicillin-resistant Staphylococcus aureus infection. J Artif Organs. 2014; 17:42–49.
11. Spicer PP, Mikos AG. Fibrin glue as a drug delivery system. J Control Release. 2010; 148:49–55.
12. Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009; 4:31–36.
13. Schrock TR, Deveney CW, Dunphy JE. Factor contributing to leakage of colonic anastomoses. Ann Surg. 1973; 177:513–518.
14. Mittermayr R, Morton T, Hofmann M, Helgerson S, van Griensven M, Redl H. Sustained (rh)VEGF(165) release from a sprayed fibrin biomatrix induces angiogenesis, up-regulation of endogenous VEGF-R2, and reduces ischemic flap necrosis. Wound Repair Regen. 2008; 16:542–550.
15. Chung YI, Kim SK, Lee YK, Park SJ, Cho KO, Yuk SH, et al. Efficient revascularization by VEGF administration via heparin-functionalized nanoparticle-fibrin complex. J Control Release. 2010; 143:282–289.
16. Yang P, Wang C, Shi Z, Huang X, Dang X, Xu S, et al. Prefabrication of vascularized porous three-dimensional scaffold induced from rhVEGF(165): a preliminary study in rats. Cells Tissues Organs. 2009; 189:327–337.
17. Woolverton CJ, Fulton JA, Salstrom SJ, Hayslip J, Haller NA, Wildroudt ML, et al. Tetracycline delivery from fibrin controls peritoneal infection without measurable systemic antibiotic. J Antimicrob Chemother. 2001; 48:861–867.
18. Andree C, Voigt M, Wenger A, Erichsen T, Bittner K, Schaefer D, et al. Plasmid gene delivery to human keratinocytes through a fibrin-mediated transfection system. Tissue Eng. 2001; 7:757–766.
19. Kanellos I, Christoforidis E, Kanellos D, Pramateftakis MG, Sakkas L, Betsis D. The healing of colon anastomosis covered with fibrin glue after early postoperative intraperitoneal chemotherapy. Tech Coloproctol. 2006; 10:115–120.
20. Woodruff MA, Rath SN, Susanto E, Haupt LM, Hutmacher DW, Nurcombe V, et al. Sustained release and osteogenic potential of heparan sulfate-doped fibrin glue scaffolds within a rat cranial model. J Mol Histol. 2007; 38:425–433.
21. Kim S, Kim Y, Hwang JW, Moon SB. Inhibitory effect of sustained perivascular delivery of paclitaxel on neointimal hyperplasia in the jugular vein after open cutdown central venous catheter placement in rats. Ann Surg Treat Res. 2017; 92:97–104.
22. Wong C, Inman E, Spaethe R, Helgerson S. Fibrin-based biomaterials to deliver human growth factors. Thromb Haemost. 2003; 89:573–582.
23. Gugenheim J, Bredt LC, Iannelli A. A randomized controlled trial comparing fibrin glue and PlasmaJet on the raw surface of the liver after hepatic resection. Hepatogastroenterology. 2011; 58:922–925.
24. Vajanto I, Rissanen TT, Rutanen J, Hiltunen MO, Tuomisto TT, Arve K, et al. Evaluation of angiogenesis and side effects in ischemic rabbit hindlimbs after intramuscular injection of adenoviral vectors encoding VEGF and LacZ. J Gene Med. 2002; 4:371–380.
25. Bauters C, Asahara T, Zheng LP, Takeshita S, Bunting S, Ferrara N, et al. Site-specific therapeutic angiogenesis after systemic administration of vascular endothelial growth factor. J Vasc Surg. 1995; 21:314–324.
26. Jozkowicz A, Fügl A, Nanobashvili J, Neumayer C, Dulak J, Valentini D, et al. Delivery of high dose VEGF plasmid using fibrin carrier does not influence its angiogenic potency. Int J Artif Organs. 2003; 26:161–169.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download