Abstract
Purpose
I introduce the use of transanal intestinal long tube (TILT) using nasogastric tube. TILT passes from anus to the anastomosis, helping to decompress a dilated bowel loop.
Methods
TILT procedure was limited to those patients predicting a severe luminal size discrepancy after intestinal anastomosis, and who had postoperative prolonged ileus. We retrospectively reviewed the medical records of 10 infants (7 male an 3 female patients) who were treated using the TILT procedure between 2012 and 2016.
Results
Median gestational age was 27+5 weeks and birth weight was 940 g. The first operation was done at a median of 4.5 days after birth due to necrotizing enterocolitis perforation (4 cases), isolated intestinal perforation (3 cases), meconium related ileus (1 case), congenital ileal volvulus (1 case), and ileal atresia (1 case). Nine cases of ileostomy closure were planned at a median of 130.5 days with a body weight of 3,060 g. For the ileal atresia case, TILT procedure without additional small bowel resection was performed to treat postoperative prolonged ileus. Nine out of ten were well functioned and defecation via anus was observed in a median of 4.5 days. Milk feeding began at a median of 6 days and the long intestinal tube was removed in a median of 14.5 days.
Conclusion
I suggested that TILT procedure could be a noninvasive operative option, predicting of size mismatched anastomosis causing prolonged ileus. Passive drainage of proximal intestinal contents might be helpful for decompress endoluminal pressure during the time of anastomosis healing with bowel movement recovery.
Pediatric surgeons are sometimes confronted with intractable small bowel obstructions after surgery for meconium related ileus, necrotizing enterocolitis, solitary bowel perforation, and gastrointestinal atresia, among others. These obstructions can be caused from intestinal adhesion, anastomosis twisting, stricture, and hypomotility caused by immaturity of enteric nervous system [12]. Intestinal anastomosis with mismatched size can be a big issue because dilated loop can compress the anastomosis leading to intestinal obstruction. It can be difficult to determine whether to wait for clinical improvement or to schedule an immediate operation. The reoperation for small infants raises many concerns about postoperative complications and recovery, even with increased surgical experience and knowledge. Here, I introduce the use of transanal intestinal long tube (TILT); a procedure arisen from complicated reoperation. TILT passes from anus to the anastomosis, helping to decompress a dilated proximal bowel loop at the first trial. In this report, I review my short experience with this new attempt for decompressing the dilated bowel.
The first attempt of TILT insertion was from complicated operation with situational complexities. The baby was born at a gestational age of 27 weeks and with a birth weight of 980 g. He underwent an initial small bowel resection with end ileostomy at 10 cm above ileocecal valve at 6 days after birth due to necrotizing enterocolitis perforation. He was referred to our clinic for an early closure of the ileostomy due to excessive water loss and ileostomy retraction. Aside from an adhesiolysis, the ileostomy closure was uneventful. Though the proximal lumen was two times greater than distal lumen, I made end to end anastomosis as usual. Unfortunately, during the 10 days postoperation, gradual aggravation and distension of the abdomen became pronounced without stool passage (Fig. 1A). We completed a second operation, creating a side to side anastomosis for decompression because the anastomosis was healthy and intact. However, this second operation did not alleviate the abdominal distension, and the bowel movement was not recovered. At 5 days post the 2nd operation, wound dehiscence occurred, along with the exposure of the peritoneum. A third operation was planned (Fig. 1B). Due to concerns of creating a stoma due to complications from the previous enterostomy, I attempted a TILT insertion. I did an ileocecectomy and inserted a 12F nasogastric tube via the anus. The tube was passed through the whole colon and anastomosis and placed 10 cm proximal to the anastomosis. The total length of the inserted nasogastric tube was 60 cm. Stool started draining well via the TILT, and abdominal distension was markedly improved. He was able to start feeding at 10 days post TILT operation and defecated via the anus. The TILT was removed 68-day postoperation due to uncertainties of this new procedure. He is doing well now without complications.
The selection criteria for the TILT procedure in stoma closure were limited to those patients showing narrow caliber of ileum and microcolon, predicting a severe luminal size discrepancy between proximal lumen and distal lumen on contrast studies. In cases where the diameter of the lumen in the distal ileum and colon was found to be less than one third of the proximal intestine during operation, we decided to attempt the TILT procedure. In addition, the TILT procedure was attempted on patients exhibiting prolonged postoperative ileus after intestinal anastomosis. If the anastomosis would be so far from ileocecal valve, or if the luminal discrepancy is not dominant, we do not do TILT procedure.
To prepare the area for a surgical drape, the entire abdomen—including the inguinal area—was disinfected. After trimming the ileostomy, we resected the ileum and made an end to end anastomosis with an interrupt suture; starting with the posterior wall of the anastomosis (Fig. 2A). In preparation for insertion, we made an additional side hole on the nasogastric tube (we have used both 10F and 12F nasogastric tubes with success) to prevent occlusion of the hole with stool. The tube was inserted via the anus (Fig. 2B) and routed through the rectum and sigmoid colon with the help of intra-abdominal guidance. The tube easily passed through the entire colon to the proximal side of the anastomosis. After the intestinal tube passed over the anastomosis, the anterior wall of the intestine was closed with an interrupt suture. (Fig. 2C) The tube was fixed in place at the buttock with one tagging suture and taped down. The infantogram post this TILT procedure is shown below (Fig. 3).
The external portion of the tube was connected with a closed bag, allowing intestinal contents to drain naturally. We frequently checked the patency of the intestinal tube by milking and gently aspirating procedures, thus preventing occluding of the lumen. Irrigation with a warm saline solution was utilized 3 days post operation when the lumen became occluded.
We retrospectively reviewed the medical records of infant who were treated using the TILT procedure between March 2012 and February 2016 at the Inje University Haeundae Paik Hospital and the Dong-A Medical Center—both located in Busan, Korea. From these medical records, data surrounding gestational age, birth weight, underlying disease causing exploratory laparotomy, the timing of the 1st and 2nd operations, the maintenance period of the intestinal tube, and the time to full enteral feeding was retrospectively collected.
A series of 10 children was enrolled in this study, comprising of 7 male and 3 female patients. Median gestational age was 27+5 weeks (range, 23+2–39+1 weeks - Nine out of the ten babies were premature) and birth weight was 940 g (range, 615–2,400 g). The first operations were done at a median of 4.5 days (range, 0–33 days) with a body weight of a median of 835 g (range, 720–2,360 g). The operations were initiated due to necrotizing enterocolitis perforation (4 cases), isolated intestinal perforation (3 cases), meconium related ileus (1 case), congenital ileal volvulus (1 case), and ileal atresia (1 case). Loop ileostomies were completed for the premature babies with solitary intestinal perforation, meconium related ileus or necrotizing enterocolitis and congenital ileal volvulus. For the case of ileal atresia, I did a resection and anastomosis (Table 1).
The second operation for ileostomy closure was planned at a median of 130.5 days (range, 86–226 days) with a median body weight of 3,060 g (range, 2,400–5,000 g). Before the 2nd operation, we performed distal contrast enemas and observed the passage of contrast in the distal ileum and colon. All of these operations were uneventful. For the ileal atresia case, postoperative obstructions were not improved over 3 weeks. We performed the 2nd operations as a TILT procedure without additional small bowel resection and anastomosis.
Nine out of ten TILT procedures were well functioned and defecation via natural anus was observed in a median of 4.5 days (range, 3–12 days). Milk feeding began at a median of 6 days (range, 2–15 days) and enteral feeding of 60 kcal/kg/day was reached at a median of 10 days (range, 5–27 days) after the TILT procedure. The long intestinal tube was removed in a median of 14.5 days (range, 3–68 days) and the time to full enteral feeding was a median of 15.5 days (range, 8–47 days) postoperatively. In 1 case, the malfunctioning tube was removed 3 days after the TILT procedure. In this patient, intestinal obstruction was prolonged for 3 days longer after the tube was removed. This was relieved after gastrograffin enema. Nine of the patients recovered well and have had no episodes of ileus. One patient with severe bronchopulmonary dysplasia died from respiratory distress with sepsis after 2 months (Table 2).
Most infants recover well from surgical treatments for intestinal anastomosis, especially with surgical technique improvements and excellent wound healing properties in infants. However, when an anastomosis leakage develops, prompt surgical treatment is needed to prevent septic complications and correct the anastomosis failure. The overall rate of anastomosis leakage ranges from 2%–12% and is correlated to morbidity and mortality [34]. Possible risk factors of anastomosis leakage have been reported as:
For pediatric surgeries, endoluminal pressure and motility are also influential. To reduce endoluminal pressure, there is always a concern with the intestinal lumen diameter and proper alignment for the anastomosis during the operation. Intestinal anastomosis for smaller diameter usually requires interrupt sutures with fine suture material in order to prevent intestinal lumen narrowing. The initial attempt for TILT was to reduce the endoluminal pressure. For the anastomosis with size discrepancy, the passage from dilated proximal loop is often delayed and finally the lumen of anastomosis is compressed with collapsed. I believe that it could be a role of drainage tube like nasogastric tube in the stomach. To relieve the pressure of proximal intestinal contents might be helpful during the time of anastomosis healing. If the motility of distal bowel is insufficient, TILT would be able to replace the bowel movement passively. As in my initial case, absence of bowel movements is disastrous, even with the confirmation of the presence of ganglion cells in the intestine. The etiology of motility disorder is mostly unclear and is extremely difficult to link in a cause-and-effect relationship between neuronal, interstitial cells of Cajal, or muscle deficits and motility dysfunction [2].
It was very helpful for intestinal atresia case. The atretic distal segment can lead to severe luminal discrepancy of anastomosis and the motility of distal segment is markedly decreased than proximal segment. We can do plication and tapering enteroplasty also. While waiting for restoring of distal bowel movement, TILT can be a bridge procedure for decompression.
Similar to the TILT procedure for infants in my experience, transanal drainage tubes have been tried in colorectal surgeries, especially low rectal cancer surgeries. It showed significant decrease of anastomosis leakage rates and prevention of severe complications, regardless of patient's factors and surgical factors [4161718]. I think one key difference in previous usage and the TILT procedure is the length of the drainage tube. Transanal drainage tube is shorter than TILT.
Actually we do not have any long intestinal tube reaching ileum from mouth. That is why I chose the tube insertion from anus. Among available commercial tubes, I tried to use a nasogastric tube. The length of the nasogastric tubes ranged from 80 to 150 cm. While this is too short to pass through the entire colon in children and adults, it is long enough for small infants weighing less than 5 kg to pass through the entire colon and into the terminal ileum via anus.
Early on in TILT procedures, I concerned about the tube remaining in the abdominal cavity and if the abdominal wall would close. However, these complications did not arise. In order to keep the tube in place, we did a single suture near the anus and taped the intestinal tube on the buttock.
In our experience, postoperative intestinal obstructions after intestinal anastomosis have occurred due to adhesive bands, anastomosis narrowing, or anastomosis folding due to proximal bowel dilation. Peritoneal adhesions are a result of peritoneal irritation by infection or surgical trauma. While the development of peritoneal adhesions has been extensively studied, there have been no definitive strategies designed to prevent them. We attempt to prevent peritoneal damage by employing gentle handling, meticulous hemostasis, and continuous irrigation, avoidance of unnecessary drying and ineffective use of foreign bodies. However, even with these prevention techniques, adhesive intestinal obstruction is unpredictable [19].
Anastomosis stricture can still cause intestinal obstructions. There are sporadic reports of balloon catheter dilations of focal intestinal strictures for small infants [202122]. It might be a safe and effective option to avoid repeat surgery that may cause intra-abdominal adhesion and short bowel syndrome [22] but it is difficult to expand the usage. To that point, TILT can also be simply utilized to maintain the patency for preventing anastomosis stricture, twisting and folding.
Herein, we introduce a simple method to decompress endoluminal pressure using a TILT. The nasogastric tube is readily available, and the simple process is applicable to decompress bowel contents with enough length and diameter for small infants. We agree that TILT procedure can be risky by causing intestinal lumen obstruction. This should not be utilized as a routine procedure for intestinal anastomosis for small infants and these trials should not replace an operation in all cases. Also, we cannot compare TILT group and non-TILT group in pediatric population. It could not prove the benefit of TILT procedure over all pediatric surgery. It requires a prospective case-control study. However, despite a small case series with a selection bias of the surgeon's subjective nature, it is worthy of consideration that could be a noninvasive operative management option, predicting of size mismatched anastomosis causing prolonged ileus.
In conclusion, we suggested that TILT procedure could be a noninvasive operative option, predicting of size mismatched anastomosis causing prolonged ileus. Passive drainage of proximal intestinal contents might be helpful during the time of anastomosis healing with bowel movement recovery.
Figures and Tables
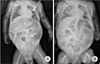 | Fig. 1(A) At 10 days postoperation, gradual aggravation and distension of the abdominal cavity became pronounced due to lack of stool passage. (B) At 5 days post the second operation, it did not show an improvement of bowel distension. |
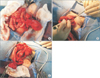 | Fig. 2(A) We made an end to end anastomosis with an interrupt suture; starting with the posterior wall of the anastomosis. (B) The tube was inserted via the anus, and passed through the entire colon to the proximal side of the anastomosis. (C) After the intestinal tube passed over the anastomosis, the anterior wall of the intestine was closed with an interrupt suture. |
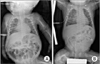 | Fig. 3The infantogram post this transanal intestinal long tube procedure is shown above. (A) Transanal intestinal long tube (TILT) was well located in the small bowel. (B) Small bowel was well decompressed via TILT after 5 days. |
References
1. Feichter S, Meier-Ruge WA, Bruder E. The histopathology of gastrointestinal motility disorders in children. Semin Pediatr Surg. 2009; 18:206–211.
2. Burns AJ, Roberts RR, Bornstein JC, Young HM. Development of the enteric nervous system and its role in intestinal motility during fetal and early postnatal stages. Semin Pediatr Surg. 2009; 18:196–205.
3. Golub R, Golub RW, Cantu R Jr, Stein HD. A multivariate analysis of factors contributing to leakage of intestinal anastomoses. J Am Coll Surg. 1997; 184:364–372.
4. Zhao WT, Hu FL, Li YY, Li HJ, Luo WM, Sun F. Use of a transanal drainage tube for prevention of anastomotic leakage and bleeding after anterior resection for rectal cancer. World J Surg. 2013; 37:227–232.
5. McArdle CS, Hole D. Impact of variability among surgeons on postoperative morbidity and mortality and ultimate survival. BMJ. 1991; 302:1501–1505.
6. Meagher AP. Colorectal cancer: is the surgeon a prognostic factor? A systematic review. Med J Aust. 1999; 171:308–310.
7. Matthiessen P, Hallbook O, Andersson M, Rutegard J, Sjodahl R. Risk factors for anastomotic leakage after anterior resection of the rectum. Colorectal Dis. 2004; 6:462–469.
8. Yeh CY, Changchien CR, Wang JY, Chen JS, Chen HH, Chiang JM, et al. Pelvic drainage and other risk factors for leakage after elective anterior resection in rectal cancer patients: a prospective study of 978 patients. Ann Surg. 2005; 241:9–13.
9. Alberts JC, Parvaiz A, Moran BJ. Predicting risk and diminishing the consequences of anastomotic dehiscence following rectal resection. Colorectal Dis. 2003; 5:478–482.
10. Vignali A, Fazio VW, Lavery IC, Milsom JW, Church JM, Hull TL, et al. Factors associated with the occurrence of leaks in stapled rectal anastomoses: a review of 1,014 patients. J Am Coll Surg. 1997; 185:105–113.
11. Makela JT, Kiviniemi H, Laitinen S. Risk factors for anastomotic leakage after left-sided colorectal resection with rectal anastomosis. Dis Colon Rectum. 2003; 46:653–660.
12. Konishi T, Watanabe T, Kishimoto J, Nagawa H. Risk factors for anastomotic leakage after surgery for colorectal cancer: results of prospective surveillance. J Am Coll Surg. 2006; 202:439–444.
13. Buchs NC, Gervaz P, Secic M, Bucher P, Mugnier-Konrad B, Morel P. Incidence, consequences, and risk factors for anastomotic dehiscence after colorectal surgery: a prospective monocentric study. Int J Colorectal Dis. 2008; 23:265–270.
14. Khoury GA, Waxman BP. Large bowel anastomoses. I. The healing process and sutured anastomoses. A review. Br J Surg. 1983; 70:61–63.
15. Matheson DM, Arabi Y, Baxter-Smith D, Alexander-Williams J, Keighley MR. Randomized multicentre trial of oral bowel preparation and antimicrobials for elective colorectal operations. Br J Surg. 1978; 65:597–600.
16. Brandl A, Czipin S, Mittermair R, Weiss S, Pratschke J, Kafka-Ritsch R. Transanal drainage tube reduces rate and severity of anastomotic leakage in patients with colorectal anastomosis: a case controlled study. Ann Med Surg (Lond). 2016; 6:12–16.
17. Shigeta K, Okabayashi K, Baba H, Hasegawa H, Tsuruta M, Yamafuji K, et al. A meta-analysis of the use of a transanal drainage tube to prevent anastomotic leakage after anterior resection by double-stapling technique for rectal cancer. Surg Endosc. 2016; 30:543–550.
18. Ha GW, Kim HJ, Lee MR. Transanal tube placement for prevention of anastomotic leakage following low anterior resection for rectal cancer: a systematic review and meta-analysis. Ann Surg Treat Res. 2015; 89:313–318.
19. Arung W, Meurisse M, Detry O. Pathophysiology and prevention of postoperative peritoneal adhesions. World J Gastroenterol. 2011; 17:4545–4553.
20. Miraglia R, Catalano P, Maruzzelli L, Riva S, Spada M, Alberti D, et al. Balloon dilatation of postoperative small bowel anastomotic stricture in an infant with apple peel intestinal atresia after serial transverse enteroplasty and jejunoileal anastomosis. J Pediatr Surg. 2010; 45:e25–e28.
21. Peer A, Klin B, Vinograd I. Balloon catheter dilatation of focal colonic strictures following necrotizing enterocolitis. Cardiovasc Intervent Radiol. 1993; 16:248–250.
22. Kim JY, Song SY, Koh BH, Cho OK, Kim Y, Park HK. Balloon dilation of postoperative small bowel stricture in an infant. J Vasc Interv Radiol. 2008; 19:1795–1796.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download