Abstract
Purpose
Neointimal hyperplasia (NH) is considered to be one of the main causes of vascular access occlusion in patients receiving hemodialysis. Endothelial injury and TGF-β-mediated proliferation of vascular smooth muscle cells (VSMCs) induce NH. Endothelial microparticles (EMPs) are also increased by endothelial injury. We aimed to investigate the effects of EMPs and TGF-β expression on VSMC proliferation and their contributions to NH formation in an ex vivo model.
Methods
EMPs were collected from the culture media of human umbilical vein endothelial cells treated with indoxyl sulfate (IS, 250 µg/mL) after ultracentrifugation at 100,000 × g. Porcine internal jugular veins were isolated and treated with EMPs (2 × 106 /mL) or left untreated for 12 days and subsequently compared with TGF-β (10 ng/mL)-treated venous tissue. Intima-media thickness and NH area were assessed using a digital program. Masson's trichrome staining and immunohistochemistry (IHC) analysis for α-smooth muscle actin, phosphorylated Akt, ERK1/2, p38 mitogen-activated protein kinase (MAPK), and Smad3 were performed on each vein sample.
Results
NH and VSMC proliferation developed to a significantly greater degree in EMP-treated veins compared to controls, with similar patterns seen in TGF-β-stimulated samples. IHC analysis demonstrated that EMPs markedly increased phosphorylation of Akt, ERK1/2, p38 MAPK, and Smad3 in areas of venous NH formation.
Vascular access failure is a critical factor impeding maintenance hemodialysis. Access failure critically affects the morbidity and mortality of end-stage renal disease patients receiving hemodialysis [12]. The leading cause of access dysfunction is a progressive venous stenosis at the juxta-anastomosis site formed as a result of neointimal hyperplasia (NH) [134]. Despite remarkable developments in vascular medicine over the past few decades, no strategy has effectively prevented NH; therefore, it is still considered as a leading pathological sign of vascular occlusive events [5]. NH is a complex process of proliferative/synthetic changes involving vascular smooth muscle cells (VSMCs) in the area of vascular injury, including the migration of VSMCs from the medial to the intimal layer, upregulation of vascular proinflammatory molecules, and progressive vascular luminal narrowing [167]. Although the chronic inflammatory condition formed in a uremic milieu can accelerate NH, the definitive molecular mechanisms that initiate NH formation, especially in uremic conditions, are poorly understood [7].
TGF-β is one of the most important pleotropic factors in tissue homeostasis, and its dysregulation leads to the pathogenesis of multiple human diseases, including cancer, autoimmune diseases, chronic renal diseases, and cardiovascular diseases [89]. Additionally, TGF-β participates in various fibrotic cardiovascular diseases through the induction of proliferation, hypertrophy, apoptosis, de-differentiation, and the migration of VSMCs [81011]. TGF-β expression is upregulated at the restenosis site, and increased TGF-β in vessel wall is closely associated with neointimal formation [1213]. Although TGF-β is regarded as a critical contributor to NH development and a therapeutic target in the prevention of vascular restenosis, the precise mechanism of the TGF-β stimulatory pathway is unclear.
Endothelial microparticles (EMPs) are small (0.1–1.0 µm) vesicles shed from endothelial cell membranes as a response to endothelial injury or apoptotic signals [1415]. Previous works have revealed that increased EMPs are closely associated with various inflammatory and vascular diseases, such as diabetes mellitus, atherosclerosis, stroke, and systemic lupus erythematosus [141516]. Furthermore, EMPs are highly induced by uremia and are closely associated with vascular dysfunction and early vascular access failure in hemodialysis patients [1718]. In order to trigger NH formation in the vicinity of a fistula, proliferative TGF-β signals must be activated by endothelialmediated VSMC stimulation.
The aim of this study was to investigate whether EMPs induced by uremic toxin lead to NH and activation of the multiple pathways downstream of TGF-β in an ex vivo model.
TGF-β was purchased from R&D Systems (Minneapolis, MN, USA). For the immunohistochemistry (IHC) assay, antibodies for phospho-Akt, phospho-ERK1/2, and phospho-p38 mitogen-activated protein kinase (MAPK) were purchased from Cell Signaling Technology (Danvers, MA, USA), and antibodies for phospho-Smad3 and TGF-β were obtained from Novus Biologicals (Littleton, CO, USA).
To generate microparticles from endothelial cells, we used human umbilical vein endothelial cells (HUVECs), purchased from Lonza (Walkersville, MD, USA). HUVECs were cultured in EGM-2 Singlequots endothelial cell culture media (Lonza) and were incubated with indoxyl sulfate (IS, 250 µg/mL) for 24 hours to induce the generation of EMPs. The supernatants were harvested and assayed immediately. Supernatants from the culture in each well were centrifuged for 10 minutes at 5,000 g at 4℃, followed by ultracentrifugation for 1.5 hours at 100,000 g at 4℃. The pellets were resuspended in phosphate buffered saline (PBS), and the absolute EMP count per tube was measured using a Trucount tube (BD Biosciences, San Jose, CA, USA). The protocol for EMP identification using fluorescent antibodies is described in Supplementary method.
Internal jugular veins were extracted from 5 female Yorkshire pigs weighing approximately 30 kg each. The details of the procedure are as follows: Experimental animals were anesthetized using xylazine, telazol, and atropine and maintained using 1%–3% isoflurane. A skillful operator marked an extraction region of the internal jugular vein, performed a skin incision, demarcated the subcutaneous tissue and muscle layers, and ligated the proximal end of the internal jugular vein with thread. Finally, cuts were made at and below the ligation site, and a 10- to 12-cm segment of vessel was extracted. The vein segment was promptly washed with culture media. The organ culture model is shown in Fig. 1. Each extracted vessel was cut into 2-cm-long pieces with the aid of sterile scissors and forceps. Then, while maintaining their unfolded morphology, each segment was fixed onto a nylon mesh (Fisherbrand, Pittsburgh, PA, USA) with sterile pins, placed in a 100-mm culture dish, covered with 30-mL culture media, and cultured for 12 days. The vessel segments were incubated in 3 different culture conditions: 30% Dulbecco's modified eagle medium (DMEM) media alone, EMPs (2 × 106/mL) + DMEM media, and TGF-β (10 ng/mL) + DMEM media. After 12 days, the vessels were preserved in 10% buffered formaldehyde solution until analysis and were subsequently compared to uncultured vessel segments.
In order to complete the morphological analysis and identification of collagen deposition in the vessel walls, each paraffin- embedded section was cut to a thickness of 5 µm and stained with hematoxylin-eosin and Masson's trichrome. Ten different sections from each vessel segment were selected for measurement of the NH area using InnerView software (InnerView Co., Seongnam, Korea). A digitalized image of each section was captured, and the neointimal and medial areas were circumscribed manually. The mean ratios of the digitally measured neointimal and medial areas were calculated in each group.
In preparation for IHC analysis, the tissue sections were deparaffinized and rehydrated. Briefly, each paraffin-embedded tissue slide was set on a dry hot plate overnight (40℃) to melt the paraffin. The tissue on each slide was dewaxed in xylene, rehydrated in a graded series of ethanol and double-distilled water, and the antigen was retrieved by high pressure. Then, the sections were washed with PBS and incubated within 3% hydrogen peroxide and 10% goat serum for 15 minutes. Next, the slides were incubated overnight at 4℃ with anti-α-smooth muscle actin (SMA), TGF-β, Akt, ERK1/2, p38 MAPK, Smad3, phospho-Akt, phospho-ERK1/2, phopho-p38 MAPK, and phospho-Smad3 antibodies. This was followed by the application of a secondary goat anti-rabbit biotinylated antibody and incubation in a hydrogen peroxidase-labeled detection system for 2 minutes. Isotype IgG was used as a negative control.
All data are expressed as mean ± standard error. One-way analysis of variance analysis was performed for comparison of multiple groups. Values of P < 0.05 were considered to be statistically significant. All experiments were repeated at least three times. All statistical analyses were performed using SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA) and Excel software packages.
After 12 days of treatment with either IS-induced EMPs (2 × 106/mL) or TGF-β (10 ng/mL), each cultured vein tissue displayed a markedly increased neointimal mass (Fig. 2A). A representative image of a Masson's trichrome-stained vessel shows increased collagen deposition in the intimal area in both EMP- and TGF-β-treated vessels after 12 days. In the same areas, the differentiated fibroblasts expressing α-SMA were massively increased in the EMP-treatment group. In a quantitative analysis, a 12-day treatment with EMPs or TGF-β resulted in remarkable expansion of the intimal area rather than the medial area (Fig. 2B). The neointima/media area ratio of the vessels treated with EMPs was more than 10 times higher than in those that were untreated (Fig. 2C). These changes were comparable to the results obtained with TGF-β stimulation.
We next investigated whether the IS-induced EMPs could activate the TGF-β signaling pathway and promote NH. IHC assays demonstrated that IS-induced EMPs significantly increased TGF-β expression in the neointima (Fig. 3A, B). Anti-TGF-β antibodies usually stain the cytoplasm. Increased EMPsinduced TGF-β expression was comparable to that induced by TGF-β stimulation. Furthermore, EMPs increased the expression of phosphorylated Akt, ERK1/2, Smad3 and p38 MAPK in neointimal areas (Fig. 3C–F). These molecules exhibited a nuclear staining pattern comparable to that of TGF-β stimulated vessels.
Our study showed that IS-induced EMPs highly influenced NH and smooth muscle cell proliferation though phosphorylation of TGF-β downstream molecules in VSMCs. TGF-β is a well-established factor in the pathophysiology of intimal hyperplasia. Animal models of artificial vascular injury have demonstrated that TGF-β administration or overexpression markedly enhanced NH at the site of vascular injury [71011]. Other studies have shown that upregulated TGF-β secretion plays a critical role in neointima formation via fibroblast recruitment, the regulation of inflammatory macrophage survival, and enhancement of matrix deposition [5192021]. Both TGF-β mRNA and protein levels have been shown to be upregulated immediately after vascular injury and sustained over 3 months [5]. Thus, the enhanced profibrotic function of TGF-β in the early phase of vascular injury may contribute to progressive matrix accumulation and eventually fibrosis during NH development [522]. Our study also demonstrated massive TGF-β-induced NH within just 12 days after vascular injury. TGF-β is a critical activator of NH, and the upregulation of TGF-β signaling cascade molecules promotes the proliferation and recruitment of VSMCs in vascular pathology models. However, the crosstalk mechanism that appears to exist between injured endothelial cells and VSMCs needs to be clarified. Our study showed that EMPs have a critical role in not only stimulating the TGF-β signaling pathway in VSMCs, but also in neointimal formation. It is not certain how EMPs activate the TGF-β signaling pathway in VSMCs. Microparticles are known to carry information both interiorly and exteriorly to communicate with almost all cell types. The mechanisms and properties of microparticles are usually determined by the surface antigens originating from their parental cells (e.g. platelets, endothelial cells, leukocytes, macrophages, and smooth muscle cells) [15]. They can activate surface receptors on target cells and regulate multiple signal processes like thrombosis, coagulation, inflammation, the immune system, and general cellular homeostasis [142324]. However, shedding microparticles can also transport specific molecular information, such as mRNA and microRNA. They can transfer this information from one cell to another cell via binding specific receptors and fusion with a target cell [142526]. In recent murine experiments, fibroblast-derived microparticles carrying TGF-β siRNA delivered siRNA to murine tumor cells, resulting in the suppression of tumor cell growth and signaling downstream of TGF-β [27]. These findings offer a possible explanation for how EMPs stimulate the TGF-β signaling pathway in VSMCs.
Subcellular TGF-β signal transduction pathways are known to be very complicated. Although the TGF-β response in all cell types is mediated primarily via a Smad-dependent pathway, it has been recently demonstrated that several Smad-independent pathways are simultaneously co-activated [282930]. The present study also showed that a 12-day EMP treatment greatly induced the overexpression of phosphorylated Smad3, ERK1/2, and Akt. This suggests that EMPs can stimulate both Smad- and non-Smad pathways.
There are some limitations in our study. First, vascular access failure occur in vessels exposed to a chronic uremic environment. An in vivo environment that induces NH differs from our ex vivo model because numerous upregulated inflammatory cytokines or oxidative stresses other than TGF-β-stimulated molecules can affect NH formation. In addition, our ex vivo models comprised healthy vessels. However, we showed that uremia-induced EMPs promoted significant NH in healthy vein wall tissue. This is an important finding given that only 12 days of EMP treatment induced remarkable NH, and uremia itself can produce a lot of circulating EMPs prior to access formation. Second, neointimal formation is affected not only by TGF-β receptor stimulation, but also by vessel wall stress generated by flow changes. We did not, however, consider flow-mediated vessel changes in the design of our study. A study examining the effects of physical wall stress along with the uremic condition should be performed. Third, how EMPs contact, trigger proliferation, and interact with the medial layer of VSMCs has not been defined. Because various other complicating factors influence NH formation in vivo, the specific effect of EMPs on NH development may be difficult to define. A proper model demonstrating how cellular membraneshed microparticles contact VSMCs could conceivably be developed.
Despite these limitations, this study highlighted a critical role for EMPs in TGF-β signal activation and neointimal formation. Although the inhibition of multiple TGF-β downstream molecules could be effective therapeutic targets in treating vascular inflammation, the development of treatments aimed at reducing EMPs generation could be more feasible. This study suggests that microparticles could be used as therapeutic tools to deliver molecules to suppress NH formation. Alternatively, the measurement of EMPs could be employed as an early indicator of impending vascular failure.
In conclusion, IS-induced EMPs provoked massive NH though activation of the TGF-β signaling pathway in VSMCs. Future study is needed to investigate how EMPs regulate VSMC proliferation and the TGF-β signaling pathway in vivo.
Figures and Tables
Fig. 1
Ex vivo vein model of endothelial microparticles (EMPs) or TGF-β induction of neointimal hyperplasia. Each vessel segment cut into 2-cm pieces was fixed onto a nylon mesh with sterile pins with care to maintain their unfolded morphology, placed in a 100-mm culture dish, covered with 30-mL culture media, and cultured for 12 days. Vessel segments were incubated in 3 different culture conditions: 30% Dulbecco's modified eagle medium (DMEM) media only, EMPs (2 × 106/mL) + DMEM media, and TGF-β (10 ng/mL) + DMEM media. After 12 days, the vessels were preserved in 10% buffered formaldehyde solution until analysis and subsequently compared to uncultured vessel segments. H&E, hematoxylin-eosin; α-SMA, α-smooth muscle actin.
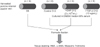
Fig. 2
Morphological analysis of ex vivo endothelial microparticle (EMP)- and TGF-β-stimulated vein. (A) Representative images of H&E (top row), Masson's trichrome (middle row), and α-smooth muscle actin (SMA) stained (bottom row) ex vivo vein (original magnification, ×100). Evaluation with one-way analysis of variance showed a significantly increased intimal area in the EMP- and TGF-β-treated groups (B) and a prominently increased intimal-medial thickness in EMP- and TGF-β-treated veins (n = 6 in each group) (C). *P < 0.05 vs. D0, ***P < 0.001 vs. D0.
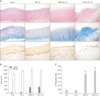
Fig. 3
Immunohistochemistry assay for TGF-β signaling molecules. Porcine veins after 12 days of endothelial microparticle (EMP) stimulation showed significant (A) neointimal hyperplasia (H&E, ×100) and revealed significantly increased expression of (B) TGF-β and phosphorylation of (C) Akt, (D) ERK1/2, (E) Smad3, and (F) p38 compared to TGF-β-stimulated veins (original magnification, ×100).

ACKNOWLEDGEMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), which is funded by the Ministry of Education, Science, and Technology (NRF-2012R1A1A3002812). This study was also supported by a Grant from the Korean Society of Nephrology (BAXTER 2014).
References
1. Roy-Chaudhury P, Sukhatme VP, Cheung AK. Hemodialysis vascular access dysfunction: a cellular and molecular viewpoint. J Am Soc Nephrol. 2006; 17:1112–1127.
2. Rothuizen TC, Wong C, Quax PH, van Zonneveld AJ, Rabelink TJ, Rotmans JI. Arteriovenous access failure: more than just intimal hyperplasia? Nephrol Dial Transplant. 2013; 28:1085–1092.
3. Heine GH, Ulrich C, Kohler H, Girndt M. Is AV fistula patency associated with angiotensin-converting enzyme (ACE) polymorphism and ACE inhibitor intake? Am J Nephrol. 2004; 24:461–468.
4. Allon M, Robbin ML. Increasing arteriovenous fistulas in hemodialysis patients: problems and solutions. Kidney Int. 2002; 62:1109–1124.
5. Jiang Z, Tao M, Omalley KA, Wang D, Ozaki CK, Berceli SA. Established neointimal hyperplasia in vein grafts expands via TGF-beta-mediated progressive fibrosis. Am J Physiol Heart Circ Physiol. 2009; 297:H1200–H1207.
6. Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. J Pathol. 2000; 190:300–309.
7. Tsai S, Hollenbeck ST, Ryer EJ, Edlin R, Yamanouchi D, Kundi R, et al. TGF-beta through Smad3 signaling stimulates vascular smooth muscle cell proliferation and neointimal formation. Am J Physiol Heart Circ Physiol. 2009; 297:H540–H549.
8. Ruiz-Ortega M, Rodriguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J. TGF-beta signaling in vascular fibrosis. Cardiovasc Res. 2007; 74:196–206.
9. Rosenbloom J, Castro SV, Jimenez SA. Narrative review: fibrotic diseases: cellular and molecular mechanisms and novel therapies. Ann Intern Med. 2010; 152:159–166.
10. Kanzaki T, Tamura K, Takahashi K, Saito Y, Akikusa B, Oohashi H, et al. In vivo effect of TGF- beta 1. Enhanced intimal thickening by administration of TGF- beta 1 in rabbit arteries injured with a balloon catheter. Arterioscler Thromb Vasc Biol. 1995; 15:1951–1957.
11. Wolf YG, Rasmussen LM, Ruoslahti E. Antibodies against transforming growth factor-beta 1 suppress intimal hyperplasia in a rat model. J Clin Invest. 1994; 93:1172–1178.
12. Zegarska J, Paczek L, Pawlowska M, Wyczalkowska A, Michalska W, Ziolkowski J, et al. Increased mRNA expression of transforming growth factor beta in the arterial wall of chronically rejected renal allografts in humans. Transplant Proc. 2006; 38:115–118.
13. Ryan ST, Koteliansky VE, Gotwals PJ, Lindner V. Transforming growth factorbeta-dependent events in vascular remodeling following arterial injury. J Vasc Res. 2003; 40:37–46.
14. Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009; 19:43–51.
15. Martinez MC, Tesse A, Zobairi F, Andriantsitohaina R. Shed membrane microparticles from circulating and vascular cells in regulating vascular function. Am J Physiol Heart Circ Physiol. 2005; 288:H1004–H1009.
16. Combes V, Simon AC, Grau GE, Arnoux D, Camoin L, Sabatier F, et al. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J Clin Invest. 1999; 104:93–102.
17. Amabile N, Guerin AP, Leroyer A, Mallat Z, Nguyen C, Boddaert J, et al. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol. 2005; 16:3381–3388.
18. Ryu JH, Lim SY, Ryu DR, Kang DH, Choi KB, Kim SJ. Association between vascular access failure and microparticles in hemodialysis patients. Kidney Res Clin Pract. 2012; 31:38–47.
19. Jiang Z, Yu P, Tao M, Fernandez C, Ifantides C, Moloye O, et al. TGF-beta- and CTGF-mediated fibroblast recruitment influences early outward vein graft remodeling. Am J Physiol Heart Circ Physiol. 2007; 293:H482–H488.
20. Heaton NS, Wolff RA, Malinowski RL, Hullett DA, Hoch JR. Antisense to transforming growth factor-beta(1) facilitates the apoptosis of macrophages in rat vein grafts. J Vasc Res. 2008; 45:365–374.
21. Khan R, Agrotis A, Bobik A. Understanding the role of transforming growth factor-beta1 in intimal thickening after vascular injury. Cardiovasc Res. 2007; 74:223–234.
22. Wu J, Zhang C. Neointimal hyperplasia, vein graft remodeling, and long-term patency. Am J Physiol Heart Circ Physiol. 2009; 297:H1194–H1195.
23. Del Conde I, Shrimpton CN, Thiagarajan P, Lopez JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005; 106:1604–1611.
24. Muller I, Klocke A, Alex M, Kotzsch M, Luther T, Morgenstern E, et al. Intravascular tissue factor initiates coagulation via circulating microvesicles and platelets. FASEB J. 2003; 17:476–478.
25. Mack M, Kleinschmidt A, Bruhl H, Klier C, Nelson PJ, Cihak J, et al. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: a mechanism for cellular human immunodeficiency virus 1 infection. Nat Med. 2000; 6:769–775.
26. Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu L, et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS One. 2008; 3:e3694.
27. Zhang Y, Li L, Yu J, Zhu D, Zhang Y, Li X, et al. Microvesicle-mediated delivery of transforming growth factor β1 siRNA for the suppression of tumor growth in mice. Biomaterials. 2014; 35:4390–4400.
28. Seay U, Sedding D, Krick S, Hecker M, Seeger W, Eickelberg O. Transforming growth factor-beta-dependent growth inhibition in primary vascular smooth muscle cells is p38-dependent. J Pharmacol Exp Ther. 2005; 315:1005–1012.
29. Miyazono K, ten Dijke P, Heldin CH. TGFbeta signaling by Smad proteins. Adv Immunol. 2000; 75:115–157.
30. Eickelberg O. Endless healing: TGF-beta, SMADs, and fibrosis. FEBS Lett. 2001; 506:11–14.
SUPPLEMENTARY METHOD
Supplementary method can be found via https://astr.or.kr/src/sm/astr-93-11-s001.pdf.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download