Abstract
Purpose
Inhibitory effect of paclitaxel on neointimal hyperplasia after open cutdown has not been elucidated.
Methods
For the control group (n = 16), silicone 2.7-Fr catheters were placed via the right external jugular vein with the cutdown method. For the treatment group (n = 16), a mixture of 0.65 mg of paclitaxel and 1 mL of fibrin glue was infiltrated around the exposed vein after cutdown. After scheduled intervals (1, 2, 4, and 8 weeks), the vein segment was harvested and morphometric analysis was performed on cross-sections.
Results
Proliferation of smooth muscle cell (SMC) was strongly suppressed in the treatment group, and the ratio of neointima to vein wall was significantly reduced in the treatment group (8 weeks; 0.63 ± 0.08 vs. 0.2 ± 0.08, P < 0.05). Luminal patency was significantly more preserved in the treatment group, and the luminal area was significantly wider in the paclitaxel-treated group compared to the control group (8 weeks; 1.91 ± 0.43 mm2 vs. 5.1 ± 0.43 mm2, P < 0.05). Mean SMC counts measured at 1 and 2 weeks after cutdown were significantly lower in the treatment group (2 weeks; 115 ± 22 vs. 62 ± 22). Paclitaxel was undetectable in systemic circulation (<10 ng/mL).
Central venous catheter (CVC) placement in pediatric patients is performed for a variety of clinical indications. Although the percutaneous approach to CVC placement has become the standard of care, the more invasive cutdown technique is occasionally required, particularly in premature infants [1]. Sequelae of the cutdown method for CVC placement include local venous deformity, such as stenosis or stricture [23]. These complications can prevent repeated CVC insertion through the same venous access site, and may eventually lead to exhaustion of readily accessible central veins.
We previously conducted a qualitative analysis of the serial histological changes in the unligated external jugular vein (EJV) wall in rats after open cutdown, including their interactions with the pericatheter sleeve. Our study demonstrated that circumferential neointimal hyperplasia in the vein and consequent luminal narrowing were caused by smooth muscle cell (SMC) activation, proliferation, and infiltration into the pericatheter sleeve [4]. Having examined the histopathological changes that lead to neointimal hyperplasia, investigation of a method to prevent this irreversible complication in a similar subject group was warranted.
Paclitaxel is an antiproliferative agent that has been demonstrated to inhibit arterial neointimal hyperplasia in various animal models. For example, paclitaxel-coated vascular grafts were shown to suppress neointimal hyperplasia in arteriovenous hemodialysis grafts in porcine models [567]. In human studies, paclitaxel-eluting stents in coronary and infrainguinal lower extremity arterial interventions have demonstrated positive clinical results in terms of in-stent restenosis and target lesion revascularization, presumably by suppressing neointimal hyperplasia [891011]. However, the impact of paclitaxel on venous neointimal hyperplasia has not been specifically addressed. In the setting of indwelling CVCs, minimizing venous neointimal hyperplasia and the resultant vascular deformity could offer the potential to preserve vital access sites for future central venous access. Thus, the purpose of this study was to evaluate the potentially protective effect of paclitaxel on EJVs in rats following open cutdown CVC placement. We performed quantitative histopathological analysis of the veins to test our hypothesis that sustained perivascular application of paclitaxel would inhibit the previously reported circumferential venous neointimal hyperplasia by suppressing SMC activation and proliferation, without associated systemic toxicity.
This study was approved by the Kangwon National University Institutional Animal Care and Use Committee. Humane care was applied in compliance with the “Guide for the Care and Use of Laboratory Animals” (National Institute of Health, publication No. 85-23, revised 1996). Eight-week-old, pathogenfree male Sprague-Dawley rats with initial weight of 250 g were purchased for the study (DBL Co., Eumseong, Korea). Diet was not restricted at any point in the investigation.
A total of 32 rats underwent CVC placement using the cutdown method. The control group (n = 16) was defined as rats that only underwent cutdown CVC placement, while the treatment group (n = 16) comprised rats that received paclitaxel after cutdown. Overall, the materials and methods utilized in this study were identical to those used in our previous investigation [4], with the exception of paclitaxel application in the treatment group of the current study.
Sterile, 2.7-Fr, single-lumen, beveled-tip silicone catheters were used (Broviac, Bard Access System Inc., Salt Lake City, UT, USA) for both the control and treatment groups. The catheters were flushed with heparin solution (100 IU/mL) before the surgical procedure.
A mixture of 0.65 mg of paclitaxel (Genexol, Samyang Genex Inc., Daejeon, Korea) and 1 mL of fibrin glue prepared according to the manufacturer's instructions (Greenplast, Greencross Co., Seoul, Korea) was prepared shortly before application to rats in the treatment group.
Prior to the operation, each rat was anesthetized with a single intraperitoneal dose of tiletamine/zolazepam (Zoletil, Virbac Laboratories, Carros, France), 20 mg/kg. A single subcutaneous dose of atropine (Daihan Pharm Co., Seoul, Korea), 0.04 mg/kg, was administered to decrease tracheal secretions. Finally, cefmetazole (Daewoong Co., Seoul, Korea), 50 mg/kg, was administered as a single intraperitoneal dose for antibiotic prophylaxis.
A vertical right paramedian incision was made after identifying the pulsating right EJV. Subsequently, the platysma muscle was divided to expose the right EJV, which was then mobilized from the junction of the facial and maxillary veins to its junction with the right subclavian vein. After securing the proximal and distal right EJV with fine vascular loops, a 20-gauge needle was used for venipuncture of the anterior wall of the EJV. Next, the beveled-tip catheter was inserted through the venipuncture and advanced to a depth of 4 cm, positioning the tip at the inferior aspect of the superior vena cava. Closure of the venipuncture was not required. After a bolus of heparin solution (100 IU/mL) was injected into the catheter to prevent catheter thrombosis, the catheter end was occluded with a titanium clip (Surgiclip; Covidien, North Haven, CT, USA). For the treatment group, the paclitaxel mixture was infiltrated around the exposed vein with 24-gauge hypodermic needle. For both control and treatment groups, the platysma layers were approximated with 6-0 absorbable sutures (Vicryl, Ethicon, Somerville, NJ, USA), while the skin was closed with a skin stapler.
The catheters were left in situ for different durations prior to histopathological assessment in order to investigate the changes in the veins over time. Catheters were removed at the following intervals: 1 week (n = 4), 2 weeks (n = 4), 4 weeks (n = 4), and 8 weeks (n = 4) postoperatively.
Once the appropriate interval for each indwelling catheter had elapsed, the animals were anesthetized in the same manner as for the surgical procedure. A long, midline incision was made from the chin to the xiphoid process, exposing the right carotid artery and left EJV, which were then cannulated with 26-gauge catheters. Heparin (1,000 IU/kg) was administered into the left EJV, followed by a bolus of 1,000-mg/kg anesthetic solution for euthanasia.
Next, 100 mL of 10% buffered formalin solution was infused at a physiologic pressure of 100 mmHg through the right carotid artery cannula (inflow) and drained by the left EJV cannula (outflow). After in situ fixation, 10 mL of 10% gelatin solution (Sigma-Aldrich, St. Louis, MO, USA) heated to 36℃ was infused into the veins via the left EJV cannula to prevent luminal collapse. The rats were then cooled for 40 minutes in a refrigerator set to 4℃.
After cooling, the entire length of the right EJV, superior vena cava, heart, and surrounding tissues were excised en bloc through a median sternotomy and immersed in formalin overnight. Following immersion fixation, we removed a 0.5-cm-thick segment of the EJV centered at the catheter entry site and the junction with the subclavian vein. The venous segments were embedded in cassettes, and stained with hematoxylin and eosin (H&E). Immunohistochemical staining included SMC-specific anti-α-actin to confirm the presence of SMCs.
All morphometric analyses were performed using a light microscope and ToupView software (ver 3.7, ToupTek Photonics Co., Zheijang, China). Neointimal hyperplasia index (NHI), patent luminal area, and SMC count measured on representative cross-sections were compared between control and treatment groups. NHI was calculated as the quotient of the area of neointimal hyperplasia divided by the total area of the vein wall on the H&E-stained cross-sections. Patent luminal area was measured from H&E-stained cross sections as the difference of catheter area subtracted from the total luminal area, reported in mm2.
Using high-power fields (×600), the number of SMC nuclei was counted in four locations in each α-actin-stained cross-section, with each location separated by approximately one-fourth of the total vein circumference. The mean counts were reported, and compared between control and treatment groups.
Blood samples were obtained from the left EJV at the time of animal euthanasia. Plasma concentration of paclitaxel in the blood samples was measured using high-performance liquid chromatography (HPLC). Of note, a threshold plasma level of 10 ng/dL has been demonstrated as the lower limit for detection of paclitaxel by HPLC [12]. Detailed description of the mechanism of HPLC is beyond the scope of this study.
All the parameters were measured as continuous variables and expressed as mean±standard deviation. Statistical analyses were performed using IBM SPSS ver. 18.0 (IBM Co., Armonk, NY, USA). Continuous variables were compared using the Mann-Whitney U-test between the control groups and treatment groups, and significance was assigned at a P-value of <0.05.
None of the subjects died or exhibited clinical signs of infection during the perioperative or observational periods.
Neointimal hyperplasia was observed in both the control and treatment groups. The greatest measured thickness of neointimal hyperplasia was consistently thicker in the control group (Fig. 1). NHI was significantly lower in the treatment group than in the control group at each measured interval in the observation period (1 week, 0.11 ± 0.04 vs. 0.34 ± 0.04; 2 weeks, 0.26 ± 0.12 vs. 0.53 ± 0.09; 4 weeks, 0.16 ± 0.07 vs. 0.54 ± 0.11; 8 weeks, 0.2 ± 0.08 vs. 0.63 ± 0.08; Fig. 2). In contrast to the treatment group, the control group demonstrated an increase in NHI over the course of the observation period.
Patent luminal area at final observation (8 weeks) was significantly larger in the treatment group than in the control group (Fig. 3). Furthermore, the measured luminal area in the treatment group was larger at all measured intervals and enlarged over the course of the observation period (1 week, 1.73 ± 0.59 vs. 0.84 ± 0.11; 2 weeks, 1.84 ± 0.54 vs. 0.91 ± 0.13; 4 weeks, 3.04 ± 0.79 vs. 2.14 ± 0.32; 8 weeks, 5.08 ± 0.43 vs. 1.91 ± 0.43; Fig. 4).
Mean SMC counts measured at 1 week and 2 weeks after cutdown CVC placement were significantly lower in the treatment group than in the control group (1 week, 113 ± 36 vs. 240 ± 21; 2 weeks, 62 ± 22 vs. 115 ± 22; Fig. 5). However, counts measured at 4 weeks and 8 weeks demonstrated no statistically significant difference between the treatment and control groups (4 weeks, 27 ± 12 vs. 37 ± 16; 8 weeks, 18 ± 5 vs. 29 ± 10). SMC counts decreased over the course of observation in both groups (Fig. 6).
The aim of our study was to evaluate the preventive effect of sustained, perivascular delivery of paclitaxel on venous neointimal hyperplasia in rats following CVC placement using the cutdown method. Compared to controls, our treatment group exhibited significantly decreased NHI, lower SMC counts, and widened luminal area throughout the observation period, confirming paclitaxel's inhibition of venous neointimal hyperplasia and attendant luminal preservation. This study is the first to report the positive effect of paclitaxel on the prevention of neointimal hyperplasia in veins, and invites further investigation to supplement our observations.
In our previous study, proliferation of SMCs was first identified 1 week after open cutdown CVC placement, stabilizing by 4-week postprocedure [4]. As shown in SMC counts in current study, SMC counts were significantly reduced in the treatment group at early postoperative period (1 and 2 weeks) when SMC proliferation was active, whereas the counts were similar between groups after postoperative 4 weeks when SMC proliferation was stabilized. Therefore, adequate treatment should include sustained delivery of paclitaxel for a duration that covers the time period for ongoing neointimal hyperplasia. Due to the substantial toxicity associated with systemic therapy, vascular applications of paclitaxel have favored local over systemic routes of delivery. For example, paclitaxel-coated and paclitaxel-eluting devices that capitalize on the local inhibitory effects on neointimal hyperplasia have been employed to augment a variety of endovascular interventions in arteries.
Yet, for open surgical procedures such as venous cutdowns, direct, perivascular application of paclitaxel may represent the only reasonable route to deliver the medicine without associated systemic toxicity. Drug-eluting bioabsorbable vascular wrap showed promising results in surgically-placed dialysis grafts in sheep [13]. Furthermore, Kwon et al. [14] and Park et al. [15] reported that perivascular delivery of antiproliferative agents such as rapamycin or paclitaxel with F-127 pluronic gel successfully inhibited neointimal hyperplasia following carotid artery injury in rats. Our study, on the other hand, employed fibrin glue as a vehicle to deliver paclitaxel over an extended period of time. Fibrin glue is composed of supraphysiological levels of key components of the coagulation cascade, including fibrinogen, thrombin, calcium, and aprotinin. Fibrin's efficacy as a tissue sealant and hemostatic agent has fostered its widespread usage by a variety surgical specialties [16]. Additionally, fibrin glue has yielded promising results as a tissue-adherent carrier for the local delivery and slow release of medications, including antibiotics, growth factors, and chemotherapeutic agents [16]. Although the in vivo pharmacokinetics of paclitaxel release from fibrin glue have not yet been defined, our observed efficacy in inhibiting neointimal hyperplasia in the absence of evidence of local toxicity such as medial wall thinning or tissue blistering suggests that fibrin glue may represent a useful alternative for sustained perivascular drug delivery. Future studies are required to elucidate the in vivo pharmacokinetics of paclitaxel delivery via fibrin glue, as well as to assess the relative perivascular and transmural concentrations of paclitaxel over time.
The typical doses of paclitaxel administered as a chemotherapeutic agent for adult malignant tumors (135 or 175 mg/m2) [17] are much larger than those reported for prevention of neointimal hyperplasia in various clinical and experimental settings [561819]. It has generally been accepted that these extremely low doses of paclitaxel for preventing neointimal hyperplasia cannot generate sufficient systemic drug concentration to produce clinical effects. Masaki et al. [20] observed effective inhibition of neointimal hyperplasia in arteriovenous hemodialysis grafts in dogs using a thermosensitive, biodegradable copolymer for sustained local delivery. In the study, 0.26–0.65 mg of paclitaxel was mixed with biodegradable polymer and applied to perivascular space. Paclitaxel concentrations from all peripheral venous blood samples were below the detection limit (10 ng/mL) of the enzymelinked immunosorbent assay. By contrast, limited data has been present regarding the use of paclitaxel in pediatric patients, and Hurwitz et al. [21] showed that paclitaxel was well tolerated in children with recurrent or progressive brain tumors, ages 4 months to 19 years, at a dosage of 350 mg/m2 every 3 weeks. Our study used doses of 0.65 mg of paclitaxel, with all measured paclitaxel concentrations in plasma from 1 to 8 weeks postoperatively also falling below the detection limit. Since the dosages employed in the present study were approximately 1,000 fold lower than those administered for chemotherapy in study of Hurwitz et al. [21], it was expected that paclitaxel would not be detected in the systemic circulation.
In conclusion, sustained perivascular delivery of paclitaxel in a mixture with fibrin glue effectively prevented neointimal hyperplasia and subsequent venous narrowing after open cutdown CVC placement in rats while maintaining systemic paclitaxel concentrations below the detectable limit.
Figures and Tables
Fig. 1
Delineation of neointimal hyperplasia (arrow) at 1 and 2 weeks postoperatively (PO) following cutdown central venous catheter placement against whole vein wall (arrowhead). Neointimal hyperplasia was consistently thicker in the control group than in the treatment group (H&E, ×40). (A) Control group, 1 week PO, (B) paclitaxel treatment group, 1 week PO, (C) control group, 2 weeks PO, and (D) paclitaxel treatment group, 2 weeks PO.

Fig. 3
(A, B) Measurement of luminal area at 8 weeks postoperatively (H&E ×40). Measured patent luminal area was larger in the treatment group than in the control group.

Fig. 4
Patent luminal area was significantly wider in the treatment group than in the control group at each measured interval in the observation period.
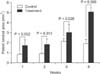
Fig. 5
Smooth muscle cell (SMC) counts in the early postoperative phases (1 and 2 weeks) measured on high-powered fields (×600) with immunohistochemical staining. SMC counts were reduced in the treatment group compared to those in the control group. (A) Control group, 1 week PO, (B) paclitaxel treatment group, 1 week PO, (C) control group, 2 weeks PO, and (D) paclitaxel treatment group, of 2 weeks PO.

ACKNOWLEDGEMENTS
This study was supported by a 2014 Research Grant from Kangwon National University (grant number: 120140657).
References
1. Hong SM, Lee HS, Moon SB. Central venous cutdown in neonates: feasibility as a bedside procedure without general anesthesia. J Pediatr Surg. 2013; 48:1722–1726.
2. Kim MJ, Chang HK, Lee MS, Han SJ, Oh JT. Internal jugular vein deformities after central venous catheterisation in neonates: evaluation by Doppler ultrasound. J Paediatr Child Health. 2010; 46:154–158.
3. Willetts IE, Ayodeji M, Ramsden WH, Squire R. Venous patency after open central-venous cannulation. Pediatr Surg Int. 2000; 16:411–413.
4. Kim S, Kim Y, Moon SB. Histological changes of the unligated vein wall adjacent to the central venous catheter after open cutdown in rats. J Pediatr Surg. 2015; 50:1928–1932.
5. Dake MD, Van Alstine WG, Zhou Q, Ragheb AO. Polymer-free paclitaxel-coated Zilver PTX Stents--evaluation of pharmacokinetics and comparative safety in porcine arteries. J Vasc Interv Radiol. 2011; 22:603–610.
6. Lee BH, Nam HY, Kwon T, Kim SJ, Kwon GY, Jeon HJ, et al. Paclitaxel-coated expanded polytetrafluoroethylene haemodialysis grafts inhibit neointimal hyperplasia in porcine model of graft stenosis. Nephrol Dial Transplant. 2006; 21:2432–2438.
7. Baek I, Hwang J, Park J, Kim H, Park JS, Kim DJ. Paclitaxel coating on the terminal portion of hemodialysis grafts effectively suppresses neointimal hyperplasia in a porcine model. J Vasc Surg. 2015; 61:1575–1582.e1.
8. Nakano Y, Ishikawa T, Mutoh M. Long-term angiographic outcomes of sirolimus- and paclitaxel-eluting stent placement in diabetes, long lesions, and small vessels. Cardiovasc Interv Ther. 2015; 30:327–337.
9. Laird JR, Hong M. Drug-eluting stents in the superficial femoral artery: The Long and Winding Road. Circulation. 2016; 133:1435–1437.
10. De Luca G, Wirianta J, Lee JH, Kaiser C, Di Lorenzo E, Suryapranata H. Sirolimus-eluting versus paclitaxel-eluting stent in primary angioplasty: a pooled patientlevel meta-analysis of randomized trials. J Thromb Thrombolysis. 2014; 38:355–363.
11. Hur SH, Cho YK, Nam CW, Kim H, Han SW, Kim YN, et al. Comparison of long-term outcomes following sirolimus-eluting stent vs paclitaxel-eluting stent implantation in patients with long calcified coronary lesions. Clin Cardiol. 2009; 32:633–638.
12. Yonemoto H, Ogino S, Nakashima MN, Wada M, Nakashima K. Determination of paclitaxel in human and rat blood samples after administration of low dose paclitaxel by HPLC-UV detection. Biomed Chromatogr. 2007; 21:310–317.
13. Kohler TR, Toleikis PM, Gravett DM, Avelar RL. Inhibition of neointimal hyperplasia in a sheep model of dialysis access failure with the bioabsorbable Vascular Wrap paclitaxel-eluting mesh. J Vasc Surg. 2007; 45:1029–1037.
14. Kwon JS, Park SS, Kim YG, Son JH, Lee YS, Kim KS, et al. Perivascular delivery of paclitaxel with F-127 pluronic gel inhibits neointimal hyperplasia in a rat carotid artery injury model. Korean Circ J. 2005; 35:221–227.
15. Park D, Kim SM, Min SI, Ha J, Kim IG, Min SK. Inhibition of intimal hyperplasia by local perivascular application of rapamycin and imatinib mesilate after carotid balloon injury. J Korean Surg Soc. 2013; 85:296–301.
16. Spotnitz WD. Commercial fibrin sealants in surgical care. Am J Surg. 2001; 182:2 Suppl. 8S–14S.
17. McEvoy GK. American hospital formulary service drug information. Bethesda (MD): American Society of Health-System Pharmacists;2000.
18. Dake MD, Ansel GM, Jaff MR, Ohki T, Saxon RR, Smouse HB, et al. Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve-month Zilver PTX randomized study results. Circ Cardiovasc Interv. 2011; 4:495–504.
19. Fernandez-Parra R, Laborda A, Lahuerta C, Lostale F, Aramayona J, de Blas I, et al. Pharmacokinetic study of paclitaxel concentration after drug-eluting balloon angioplasty in the iliac artery of healthy and atherosclerotic rabbit models. J Vasc Interv Radiol. 2015; 26:1380–1387.e1.
20. Masaki T, Rathi R, Zentner G, Leypoldt JK, Mohammad SF, Burns GL, et al. Inhibition of neointimal hyperplasia in vascular grafts by sustained perivascular delivery of paclitaxel. Kidney Int. 2004; 66:2061–2069.
21. Hurwitz CA, Strauss LC, Kepner J, Kretschmar C, Harris MB, Friedman H, et al. Paclitaxel for the treatment of progressive or recurrent childhood brain tumors: a pediatric oncology phase II study. J Pediatr Hematol Oncol. 2001; 23:277–281.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download