Abstract
Ultrasound can be an effective alternative to computed tomography for surveillance following endovascular aneurysm repair (EVAR). Recently, ultrasound fusion imaging with the real-time navigation system was introduced. Here we described 3 patients who underwent post-EVAR surveillance using this novel technique. Complete coregistration was achieved in all patients. The origin of left renal artery was selected for the target of coregistration. Ultrasound fusion imaging was useful to differentiate the confusing lesion and to evaluate the complete resolution of endoleak and newly developed delayed endoleak. Ultrasound fusion image with real-time navigation system can be a feasible imaging tool for post-EVAR surveillance.
Endovascular aortic aneurysm repair (EVAR) offers the advantage of lower perioperative morbidity and mortality. But, secondary intervention may be needed due to innate complications of EVAR procedure, such as aneurysm growth, endoleak, device migration, or structural failure [1]. Therefore, life-long surveillance to detect these complications is recommended [2].
Computed tomogram angiography (CTA) remains the most widely used imaging modality for post-EVAR surveillance because of its short examination time, reproducibility, and spatial and contrast resolution [23]. However, multiple CTA examinations can pose a radiation hazard and potential risk for contrast-induced nephrotoxicity. Ultrasound (US) can be an effective alternative to CTA since it has no risk of radiation and nephrotoxicity. But, inherent disadvantages of US include long examination time, operator dependence, and unfeasibility for obese patients.
Recently, a virtual navigation system that fuses real-time US with reconstructed CT imaging was introduced [4]. This fusion image enables definitive surveillance after EVAR without the need for new CT imaging by combining the postoperative CT scan with real-time US in a dual image display. Here, we report three cases in whom this real-time US navigation system was used for post-EVAR surveillance.
All examinations were performed using the LOGIQ E9 US system (GE Healthcare, Milwaukee, WI, USA) equipped with an electromagnetic tracking system, transmitter, and a small sensor mounted on the US probe. The sensor provides the position and orientation of the transducer in the transmitter's spatial volume (Fig. 1).
Real-time image fusion was achieved as follows. Three-dimensional CT volume data taken within 1 week after EVAR were stored as Digital Imaging and Communications in Medicine files. The data were input into the US system. Spatial coregistration was achieved to ensure that the pixels from the CT datasets represented approximately the same volume for the completion of imaging fusion. While US and CT images were displayed side-by-side, spatial coregistration was made at the origin of the left renal artery. After the complete coregistration, US scanning was performed from the level of the superior mesenteric artery to the iliac artery. Detailed scanning was done to detect the any types of endoleak, device migration, and structural failure of the device. The maximal diameter of aneurysm sac was measured at the perpendicular axis of aorta. This study was approved by the Institutional Review Board (Kyung Hee University Hospital at Gangdong, KHNMC 2016-08-032).
An 89-year-old male patient presented with 5.9-cm-sized, asymptomatic abdominal aortic aneurysm (AAA). EVAR was performed using Zenith (Cook Medical, Bloomington, IN, USA) endograft without any complications. Surveillance using US and CT fusion image was performed for postoperative 15 months. Reference CTA volume data was taken on the third postoperative day. Image fusion was made using the CT dataset (Fig. 2). There was a filling defect in the reference CT image with white color shown in Fig. 2B. It could not be differentiated with endoleak or aneurysmal sac content. It was revealed as aneurysmal sac content on fusion US image shown in Fig. 2A.
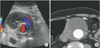
A 73-year-old male patient with 5.6-cm-sized AAA was referred. EVAR was performed using Zenith endograft without any complications. Abdominal CTA was performed to evaluate the postprocedural complications on the second postoperative day. Type II endoleak from the inferior mesenteric artery (IMA) was detected. Surveillance using US fusion imaging was done at 10 months postoperatively (Fig. 3). The fusion image completely matched (Fig. 3). The inferior vena cava was in the same position in the US and CT images. Follow-up US fusion image revealed the resolution of endoleak.
A 71-year-old male patient presented with a 9.5-cm-sized, asymptomatic AAA. EVAR was performed using Zenith endograft without any complications. On the second postoperative day, CTA was performed to evaluate postprocedural complications. There was no endoleak on this initial CTA. At 24 months postoperatively, US fusion image surveillance was performed (Fig. 4). US revealed the newly developed type III endoleak (Fig. 4A).
In this case series, US fusion imaging was a feasible option for the post-EVAR surveillance. Complete coregistration was achieved in all patients. The origin of left renal artery was selected for the target of coregistration. In the first case, CT scan showed the filling defect between the aneurysm wall and endograft. This could be interpreted as an endoleak or aneurysmal sac content. However, US fusion imaging at the same level of CTA revealed the lesion as aneurysmal sac content on the basis of absence of a color signal. In the second case, the US fusion image showed the complete resolution of type II endoleak that was apparent on CTA at the same location. In the third case, the delayed endoleak was revealed with US fusion imaging. With this imaging technique, it was possible to classify the type of endoleak because the reference CTA showed the exact position of US probe.
The advantages of fusing the different imaging modalities on the same screen in real time include theoretically improved diagnostic accuracy, which is mainly useful for image-guided interventions because biopsy or intervention is more readily performed in real time. It has been used to percutaneous radiofrequency ablation for the hepatocellular carcinoma [5]. This image modality can dramatically improve the feasibility of radiofrequency ablation treatment for hepatocellular carcinoma nodules that are not revealed on sonography [5].
Recently, a virtual needle-tracking system (VirtuTRAX CIVCO Medical Solutions, Kalona, IA, USA) was introduced in which a magnetic sensor is attached in the distal tip of an ablation needle [6]. Because the system is able to virtually visualize the tip of the needle and the needle path on US, it allows targeting from any angle and makes it easy to monitor and control the procedure. The combined use of real-time image fusion and this needle-tracking system can improve the therapeutic feasibility of ablation. Although this technique has not been used in the treatment of endoleaks, such treatment is feasible with direct injection of embolic material into the aneurysm sac under real-time US fusion image guidance.
Contrast-enhanced US (CEUS) has emerged as an alternative modality for post-EVAR surveillance [7]. It has become an essential part of post-EVAR surveillance, with very good diagnostic performance, absence of renal impairment, and no radiation, accompanied by low cost [7]. A comparison of the diagnostic accuracy between CEUS and CTA in detecting changes in AAA size and endoleaks found that CEUS demonstrated significantly more endoleaks, predominantly of type II, compared with CT angiography (53% vs. 22% of cases) [8]. US was as accurate as CT angiography in the assessment of maximal aneurysm sac diameters. The interobserver variability for AAA size measurement by US was low, given the interclass correlation coefficients of 0.99 and 0.98 for anteroposterior and transverse maximal diameters, respectively. In spite of nonexistent report of the application of contrast in US fusion image in EVAR surveillance, it seems to be more objective and accurate. The Society for Vascular Surgery recommends that US is an alternative to CTA for annual postoperative surveillance if neither endoleak nor AAA enlargement is documented during first year after EVAR [2]. Therefore, US fusion image might be used for annual postoperative surveillance.
Coregistration is the most important step in US fusion imaging. To fuse medical imaging information obtained from different modalities at different times, spatial coregistration is mandatory to ensure that the pixels from the various datasets represent approximately the same volume. There are several methods to achieve this goal depending on the imaging modality. One method to coregister 2 datasets is to define a series of standard registration points, in which external material is placed on the patient, such as fiducials or internal structure [9]. When the markers are anatomic, for instance, vessels or specific points in organs, the images have to be in high spatial resolution to assure that the same point is marked on each image [10].
In conclusion, US fusion imaging with real-time navigation system can be used to evaluate procedure-related complications and measure aneurysm diameter after EVAR. This can be a feasible imaging tool for post-EVAR surveillance.
Figures and Tables
 | Fig. 1Ultrasound system. This system was equipped with an electromagnetic tracking system, a transmitter and a small sensor mounted on the ultrasound probe. |
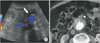 | Fig. 2Ultrasound fusion imaging can differentiate the confusing lesion. It was revealed with aneurysmal sac content on ultrasound fusion image (A; white arrow). A filling defect could not be differentiated with endoleak or aneurysmal sac content in reference CT image (B; open arrow). |
 | Fig. 3Ultrasound fusion imaging reveals the complete resolution of endoleak. Follow-up ultrasound fusion imaging reveals the complete resolution of endoleak (A) shown in initial CT scanning (B, open arrow). Fusion image was completely matched. Images of inferior vena cava was located in the same position (small arrows). |
References
1. Stroupe KT, Lederle FA, Matsumura JS, Kyriakides TC, Jonk YC, Ge L, et al. Cost-effectiveness of open versus endovascular repair of abdominal aortic aneurysm in the OVER trial. J Vasc Surg. 2012; 56:901–901.e2.
2. Chaikof EL, Brewster DC, Dalman RL, Makaroun MS, Illig KA, Sicard GA, et al. The care of patients with an abdominal aortic aneurysm: the Society for Vascular Surgery practice guidelines. J Vasc Surg. 2009; 50:4 Suppl. S2–S49.
3. Walker TG, Kalva SP, Yeddula K, Wicky S, Kundu S, Drescher P, et al. Clinical practice guidelines for endovascular abdominal aortic aneurysm repair: written by the Standards of Practice Committee for the Society of Interventional Radiology and endorsed by the Cardiovascular and Interventional Radiological Society of Europe and the Canadian Interventional Radiology Association. J Vasc Interv Radiol. 2010; 21:1632–1655.
4. Pfister K, Schierling W, Jung EM, Apfelbeck H, Hennersperger C, Kasprzak PM. Standardized 2D ultrasound versus 3D/4D ultrasound and image fusion for measurement of aortic aneurysm diameter in follow-up after EVAR. Clin Hemorheol Microcirc. 2016; 62:249–260.
5. Toshikuni N, Tsutsumi M, Takuma Y, Arisawa T. Real-time image fusion for successful percutaneous radiofrequency ablation of hepatocellular carcinoma. J Ultrasound Med. 2014; 33:2005–2010.
6. Tomonari A, Tsuji K, Yamazaki H, Aoki H, Kang JH, Kodama Y, et al. Feasibility of the virtual needle tracking system for percutaneous radiofrequency ablation of hepatocellular carcinoma. Hepatol Res. 2013; 43:1352–1355.
7. Cantisani V, Grazhdani H, Clevert DA, Iezzi R, Aiani L, Martegani A, et al. EVAR: Benefits of CEUS for monitoring stent-graft status. Eur J Radiol. 2015; 84:1658–1665.
8. Ten Bosch JA, Rouwet EV, Peters CT, Jansen L, Verhagen HJ, Prins MH, et al. Contrast-enhanced ultrasound versus computed tomographic angiography for surveillance of endovascular abdominal aortic aneurysm repair. J Vasc Interv Radiol. 2010; 21:638–643.
9. Yu H, Fahrig R, Pelc NJ. Co-registration of x-ray and MR fields of view in a hybrid XMR system. J Magn Reson Imaging. 2005; 22:291–301.
10. Goerres GW, Schuknecht B, Schmid DT, Stoeckli SJ, Hany TF. Positron emission tomography/computed tomography for staging and restaging of head and neck cancer: comparison with positron emission tomography read together with contrast-enhanced computed tomography. Clin Imaging. 2008; 32:431–437.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download