Abstract
Purpose
This study aims to examine and compare the effects of immunosuppressant cyclosporine A (CsA) and tacrolimus (TAC) on colon anastomosis recovery.
Methods
Forty rats were randomly divided into 4 groups. The 4 groups were determined as follows: control group; sham group, given %0.09 NaCl; TAC group, given 0.5 mg/kg/day tacrolimus; and CsA group, given 5 mg/kg/day CsA. A 6-cm midabdomen incision was performed on the rats. An incision of all layers on the right colon was performed. Then anastomosis was undertaken. Laparotomy was performed on the seventh day postoperation. The colon bursting pressures were evaluated, histopathological examinations were undertaken, and E-cadherin expression and tissue hydroxyproline levels were evaluated.
Results
Statistically significant differences were observed among bursting pressures of the groups (P < 0.001). The value was significantly low in TAC and CsA groups when compared to control and sham groups (P < 0.05). The tissue hydroxyproline levels were significantly low in TAC group compared to control group (P = 0.03). Fibroblast density and neovascularization were significantly greater in the control group compared to the TAC group (P < 0.05). Levels of collagen had decreased significantly in TAC group compared to other groups (P < 0.05).
Conclusion
Our study showed that TAC may have a negative effect of colon anastomosis recovery. The lowest anastomosis bursting pressure was detected in TAC group. Also, collagen, hydroxyproline, fibroblast, neovascularization and E-Cadherin levels were comparatively lower in TAC group. CsA did not cause any significant changes to tissue hydroxyproline, collagen, fibroblast, and E-Cadherin levels.
Due to the increase in organ transplants and autoimmune diseases, usage of immunosuppressive drugs is gradually increasing. Patients occasionally require operations and invasive actions whist taking immunosuppressive drugs. In such cases, there may be the need to completely cease immunosuppressive treatment, decrease the immunosuppressive treatment, or change the immunosuppressive agent preoperation. It is crucial that the balance between allograft protection and wound recovery is well kept.
Current practice following solid organ transplants is to combine a calcineurin inhibitor such as cyclosporine A (CsA) or tacrolimus (TAC), an antiproliferative agent like azathioprine or mycophenolate mofetil, with steroids and apply them as immunosuppressant drugs [1].
As a calcineurin inhibitor, TAC (FK-506, Fujimycin) decreases interleukin (IL)-2 production of T cells with concurrent effects on leucocyte apoptosis and degranulation. TAC tumor necrosis factor-α also decreases cytokines such as IL-1, -3, -4, -5, -6, and -8 [2]. CsA is another calcineurin inhibitor that decreases T-cell activity; moreover, it also decreases cyclosporine and neutrophil chemotaxis at TAC inflammation [3].
Even though immunosuppressive drugs are generally acknowledged to negatively effect wound recovery, scientific evidence to prove such is still insufficient. In a study on renal transplant receivers, wound recovery complications in different immunosuppressive protocols were determined in about 7%–53% of cases [1]. Studies also show that TAC can have both positive and negative effects on wound recovery [4]. A study conducted with CsA showed that cyclosporine has a negative effect on colon anastomosis recovery. Everolimus, an mammalian target of rapamycin (mTOR) inhibitor, has been proven to alter the wound recovery period of fascia and intestines independently of time and dosage [5].
Colonic anastomosis is one of the major procedures of general surgery [6]. Colonic anastomosis leakages are still evaluated as major complications in surgery. Anastomosis leakages cause an increase in postoperative morbidity and mortality rates
Even though there are a number of studies on the effect of TAC on intestine anastomosis recovery, the number of studies examining the effect of CsA on intestine anastomosis are limited [12]. Whilst studies comparing the effects of the most commonly used immunosuppressive agents, TAC and CsA, on anastomosis recovery have been published, this study focuses on the evaluation of colon anastomosis cases after a short-term application of immunosuppressive drugs [12]. In this study, immunosuppressive drugs were administered in the preoperative period for 14 days. What distinguishes our study from others in the field is the fact that this research was conducted on rats that had been exposed to immunosuppressive drugs during the operation.
This study aims to examine and compare the effects of immunosuppressant CsA and TAC on colon anastomosis recovery.
Forty Wistar albino rats (260–290 gr) of 12 weeks of age were randomly divided into 4 groups, 10 per group. Groups were determined as follows: control (group 1, n = 10); sham (group 2, n = 10) given %0.09 NaCl; TAC group (group 3, n = 10), given 0,5 mg/kg/day tacrolimus; CsA group (group 4, n = 10), given 5 mg/kg/day cyclosporine A.
No pharmaceutical agents or fluids were given to the rats in group 1, pre- or postoperation. Rats in groups 2, 3, and 4 were administered drugs 2 mL intraperitoneally for 7 days preoperation and 7 days postoperation. The drugs administered to rats in groups 2, 3, and 4 were: 0.09% NaCl to group 2; 0,5 mg/kg/day tacrolimus (Prograf intravenous, Astellas Phama Inc., Tokyo, Japan) within 2 mL 0.09% NaCl to group 3; 5 mg/kg/day cyclosporine A (Sandimmun iv, Novartis, Basel, Switzerland) within 2 mL 0.09% NaCl to group 4.
The drug dosages were calculated according to data found in the cited literature [78]. While TAC is toxic in high doses, 0.1–10 mg/kg/day dosages of TAC have been administered for different purposes and its use reported in various studies. Due to its toxicity in higher doses, it is preferable that this treatment be performed on rats. The intraperitoneal route was the preferred method of administration [9].
Rats were kept in a room at a controlled temperature of 18℃–24℃ and were exposed to cycles of 12 hours daylight and darkness. Four to 6 rats were kept in a single cage with free access to water and food. No food was given to the rats in the last 6 hours prior to surgery. Anesthesia was also given, in the forms of intraperitoneal ketamine (Ketalar, Pfizer, Istanbul, Turkey), 75 mg/kg; and intraperitoneal Xylazine, 10 mg/kg (Rompun, Bayer AG, Leverkusen, Germany). The operation site was shaved, cleaned and isolated with a sterile towel. All operations were completed under aseptic conditions and for this polyvinylpyrrolidone (polyvidon iodine, 10%) was used. Researchers used sterile gloves throughout the procedure and sterile surgical instruments were used in the experiment. Ceftriaxone 100 mg/kg was used for intramuscular prophylaxis purposes. Bupiviocaine (0.25%) was applied onto the surgical incisions for all rats in order to control postoperative analgesia. Postoperative analgesia was performed with ketoprofen (Profenid, Sanofi Aventis, Pari, France) 3 mg/kg subcutaneously, daily for 3 days. Each rat was given 10 mL (0.09%) subcutaneous NaCl due to fluid loss. Rats were placed into different cages after the surgery and monitored.
The surgeon who performed the anastomosis was blind to the treatment conditions. Six-centimeter midabdomen incisions were performed on all of the rats. The right colon was isolated and the blood supply was preserved and a cut of all layers was performed at the 1-cm end of the ileocecal valve. Following this, a single layer end-to-end anastomosis was applied between proximal and distal ends with 6/0 PDS (PDS II [polydioxanone] Suture, Ethicon, Somerville, NJ, USA) atraumatic suture. After each operation, the abdominal incisions of each rat were sealed with continuous stitches using 4/0 polyglactin (vicryl rapide, polyglactin, Ethicon) suture. Rats were provided with access to food and water 8 hours after the operation.
On the 7th day postoperation, intraperitoneal ketamine (Ketalar, Pfizer) (75 mg/kg) and intraperitoneal Xylazine (10 mg/kg) (Rompun, Bayer AG) were administered to each rat prior to conducting laparotomy with a left paramedian cut. For each rat the anastomosis line was isolated and the right colon was excised in a way that included the anastomosis line. After the excision, the anesthetized rats were euthanatized by decortication. For each rat the right colon distal end was closed with 3/0 silk suture. An 18F tube was placed in the proximal end and detected with 3/0 silk suture. The tip of the tube was connected to a monitor (Philips Sure Signs VM8, Philips Medical Systems, Eindhoven, The Netherlands) that simultaneously gives pressure and measures pressure in mmHg. Following this, the colon was dipped into a container filled with water and the pressure was gradually increased. The pressure value at the moment of air leakage from the anastomosis line was detected and recorded as the bursting pressure. Resection was 0,5 cm distal and proximal of the anastomosis line, and one piece of it was detected with 1-mL physiological serum. The sample tissue was stored at -80℃ until hydroxyproline appointment was finalized. The rest of the tissue was preserved in formaldehyde for histopathologic examination.
At the beginning of the study the rats were weighed and monitored daily. Measurements were recorded to compare their weights.
The hydroxyproline content of the tissues was determined using Hydroxyproline Assay Kit from Sigma (Cat. No: MAK008; St. Louis, MO, USA). The assay method is based on the reaction of oxidized hydroxyproline with 4-dimethylaminobenzaldehyde (DMAB) to give a colorimetric product at 560 nm. One hundred microliter of water was added to 10 mg of wet tissue and homogenization was performed by a sonicator equipped with a microtip (Bandelin, Berlin, Germany) at an amplitude of 25% (0.7 son, 0.2 s off cycle). Sonication continued until the tissue suspension became completely homogeneous, which typically took approximately 1–2 minutes of the total sonication time. One hundred microliter of homogenized tissue sample was mixed with 100 µL of concentrated HCl (12 M) and the mixture was hydrolyzed at 120℃ for 3 hours. After the hydrolysis was completed, samples were centrifuged at 13.000 g for 10 minutes and 20 µL of each hydrolysate was transferred into a 96-well plate. The plate was incubated at 60℃ for 3–4 hours in order to dry the samples completely. This was followed by the addition of 100-µL chloramine T solution to each well and incubation for 5 minutes at room temperature for the oxidation of hydroxyproline. Afterwards, 100 µL of DMAB reagent was added into the wells and the plate was incubated at 60℃ for 90 minutes. A microplate reader (Multiskan GO, Thermofisher Scientific Inc., Waltham, MA, USA) was used to measure absorbance at 560 nm immediately after incubation. Hydroxyproline standards with a known concentration were also measured in a similar way to the samples. The amount of hydroxyproline content was calculated using a standard curve generated from the absorbance values of the standards. Results were expressed as micrograms of Hydroxyproline per milligram of wet tissue (µg/mg).
Histopathological examinations for each rat were performed by the same pathologist, and the pathologist's analysis of the tissues was conducted blind. Pieces of tissue were prepared on a paraffin block and thin slices were examined under a light microscope with the help of hematoxylon-eosin. The resulting images were recorded on the computer. Histopathological staging was done according to the Ehrlich-Hunt model [10]. The evaluation criteria were determined with regard to the following: inflammatory cell; fibroblast; neovascularization and collagen amount. Cellular and histopathological scoring was evaluated in 4 semi-quantitative stages. Calculations were made separately for each criterion - inflammatory cell, fibroblast proliferation, neovascularization, and collagen deposition.
Sample cuts from all rats in the various study groups and the control group were taken for immunohistochemical examination. Cuts were treated with formalin fixation, paraffin application and blocking, followed by immunohistochemical coloring. E-Cadherin protein amounts were evaluated semiquantitatively in the tissues belonging to separate groups. In these tissues of different groups, the level of E-Cadherin was semiquantitatively determined (absent, 0; slight, 1 [up to 20% positive]; moderate, 2 [21%–50% positive]; potent, 3 [51%–100%]) [11].
SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. Kruskal-Wallis test was applied to group comparisons. Kruskal-Wallis post hoc tests were applied through Sidak-Dunn test in duo comparisons. Analysis of variance was used in comparing the quantitative parameters. Tukey test was used in multiple comparisons. Results were expressed with a confidence interval of 95%. Results were given mean ± standard error. Values P < 0.05 were considered significant. Median and Q1 and Q3 percentiles were calculated from determining statistics. Group size calculation was performed by the resource equation method.
During the experiment, 1 rat from groups 1, 2, and 4 died, as did 2 rats from group 3. Anastomotic leak was seen in one rat in groups 2, 3, and 4. These rats were excluded from the study.
No significant differences were observed in the comparisons for inflammatory cell (P > 0.05). Fibroblast density and neovascularization showed a significant increase in the control group compared to TAC group (P < 0.05). Collagen amounts showed a significant decrease in TAC group compared to other groups (P < 0.05) (Figs. 1, 2).
Average anastomosis bursting pressure was 115.6 ± 5.3 mmHg in the control group, 120.8 ± 4.3 mmHg in sham group, 66.2 ± 3 mmHg in TAC group, and 91.1 ± 4 mmHg in CsA group. There were statistically significant differences among bursting pressures of groups (P < 0.001). The value was significantly lower in TAC group compared to control and sham groups. (P < 0.001). The value was also significantly lower in CsA group compared to control and sham groups (P < 0.05) (Fig. 1). No significant difference was observed between TAC and CsA groups (P > 0.05).
In comparisons performed among the groups, the pattern of positive staining with E-cadherin was found to be statistically higher in control, Sham and CsA groups in contrast to TAC group (P < 0.01) (Fig. 1).
A significant statistical difference was observed among the groups in terms of tissue hydroxyproline levels (P = 0.034). A significant difference was only detected between TAC and control groups in multiple comparisons (P = 0.03). Tissue hydroxyproline levels were significantly lower in TAC group compared to control group.
Usage of immunosuppressive drugs is gradually increasing, due to the increase in organ transplants and autoimmune diseases. Operations and invasive actions may sometimes be needed during immunosuppressive drug usage. Decreasing or completely stopping immunosuppressive treatment or changing the immunosuppressive agent may be required in the preoperational period. It is crucial that a balance between protecting the allograft and healing of a wound is achieved.
Anastomosis leakages are among the most critical complications of surgery. While some chemotherapeutic agents tamper with colorectal anastomosis recovery, some agents like insulin-like growth factor promote colon anastomosis recovery [12]. This is why it is very important that the effects of TAC and CsA, 2 major immunosuppressive agents contemporaneously applied in colon anastomosis healing, are known.
TAC is an immunosuppressive agent commonly used for postorgan transplant rejection prophylaxis. It is also used in inflammatory intestine disease treatments. Preclinical studies on TAC's effect on wound recovery are controversial. While there are many studies that TAC has a positive effect on wound recovery, there are also studies stating that it tampers with the recovery process [24]. In his study, Schaffer et al. [13] also show that TAC has different effects on different tissues. While TAC was proven to tamper with dermal wound recovery in rats, no negative effects were detected for colon anastomosis recovery. In an experimental work, TAC was applied for a short time and an evaluation was concluded in the early stages of wound recovery. In another study, TAC was only applied after the operation [1]. In our study, immunosuppressive drugs were applied in the preoperational period and continued for 14 consecutive days. Our study was conducted on rats exposed to immunosuppressive drugs during the operation. This is the main difference of our study and other studies.
CsA is another calcineurin inhibitor that reduces T cell activity [14]. CsA is generally used for postorgan transplant rejection prophylaxis as well as a treatment for diseases such as atopic dermatitis and rheumatoid arthritis. Cyclosporine is an effective immunosuppressive agent [15]. Even though it is commonly used in modern times, there are a limited number of studies available evaluating its effect on the colon anastomosis recovery process. This is why CsA was chosen as the second immunosuppressive agent in our study.
Anastomosis recovery is evaluated in histopathological, biochemical, and mechanical ways. Phases of wound recovery are examined in histopathologic evaluation. Anastomosis recovery is directly related with the quality of newly synthesized collagen. Biochemical evaluation can be conducted with the help of hydroxyproline content that reflects collagen synthesis. Mechanical evaluation can on the other hand be performed with the bursting pressure of the anastomosis [16]. Histopathological, biochemical, and mechanical evaluations were conducted in our study.
In our study, bursting pressure was calculated to evaluate anastomosis strength. Bursting pressure shows the mechanical strength of anastomosis [17]. Bursting pressures in TAC and CsA groups were significantly lower than in control and isotonic groups. This result proves that both TAC and CsA decrease the mechanical strength of anastomosis. The most prominent parameters to affect anastomotic strength are collagen deposition amounts and cross-linking of the collagen. Collagen deposition also reflects collagen synthesis and breakdown [4]. In our study, a decrease in collagen deposition was detected in TAC group. Decreasing collagen can be related with the decreasing bursting pressure levels, which shows the mechanical strength of anastomosis. Even though there was a significant decrease of bursting pressure in CsA group compared to control and isotonic groups, no significant difference in collagen deposition was observed. This may be related to the other important factor that affects anastomosis strength: cross-linking of collagen. Besides, mechanical strength of the anastomosis is also related to the structural collagen web in the submucosal layer of the colon [18]. Decrease in the submucosal granulation tissue on suture line also affects anastomosis strength [19]. Decreased fibroblast activity, collagen deposition, and neovascularization in TAC group were determined to be related to the decrease in granulation tissue. Decreasing anastomosis tissue may be related to the decrease in anastomosis strength. Bursting was from the anastomosis line in the majority of the rats in the CSA and TAC groups. Bursting was also seen in the non-anastomotic area in the control and sham groups. Bursting in the anastomotic line seen in CSA and TAC groups can be due to a decrease of mechanical anastomosis strength. Mechanical anastomosis strength can be associated with the amount of collagen deposition and collagen cross-linking.
Patient's feeding patterns tend to be affected after gastrointestinal surgery. Patients may show signs of malnutrition and anastomosis strength may be decreased due to collagen deposition decrease [20]. In a study, rats exposed to TAC were observed to be in a more catabolic state. Although a significant decrease in rats' weights was determined, wound recovery processes were not affected negatively [1]. In our study, a significant weight loss in the group of TAC rats was determined compared to other groups at the end of the experiment. The decrease in collagen deposition caused by malnutrition may be one of the reasons for the decrease in anastomosis bursting pressures in the TAC group.
Inflammatory cell infiltration includes the second phase during anastonomosis recovery process. Chemotactic agents, cytokines and inflammatory cells are densely found in the anastomosis area on the 2nd day postoperative [21]. An experimental study shows that the inflammatory cell density in the anastomosis area is related to the postoperative day count [22]. No significant difference was observed between inflammatory cell densities of different groups in our study. This may be caused by the fact that histopathologic samples were not taken in the early postoperative period, when inflammatory cell changes are at the peak.
In our study, a significant decrease in neovascularization in the TAC group was detected when compared to other groups. CsA group on the other hand did not show any significant difference compared to control and isotonic groups. Neovascularization is of utmost importance in anastomosis recovery period. Good blood build up in the anastomosis area contributes to anastomosis recovery. An experimental study showed that intraperitoneal or topical application of TAC suppresses vascular endothelial growth factor (VEGF) and causes an anti-VEGF effect. VEGF is a very important factor for angiogenesis [23]. CsA on the other hand causes an increase in neoangiogenesis. Also, CsA decreases the inflammatory response [24]. Even though an increase in neovascularization in CsA group was detected compared to TAC, no significant statistical value was recorded.
Fibroblast plays an active role in collagen synthesis and extracellular matrix build up during the wound recovery process [25]. Fibroblast is very important for wound strength. A study conducted on TAC's effect on scar formation shows that there was a decrease in fibroblast density in the group exposed to TAC. The study also shows that this effect was related to fibroblast apoptosis developed due to TAC activating c-JUN N-terminal kinase and extracellular signal-regulated kinase [26]. Our study also showed that there was a significant decrease in fibroblast density in TAC group compared to control group. Low collagen amounts and bursting pressure in TAC group are thought to be related to the decreased fibroblast count. In another study, Janikowska et al. [27] show the proliferative effect CsA has on human dermal fibroblasts. No significant difference in the fibroblast density of CsA group in contrast to other groups was observed.
E-cadherin is one of the molecules responsible of adhesion and communication between cells. An increase in E-cadherin expression causes an increase in cell-to-cell adhesion. Cellular adhesion molecules are shown to play an important role in the peritoneal adhesion formation process [28]. In our study, TAC group showed a significant decrease in E-Cadherin expression compared to other groups. There was not adequate data for this obtained result to be discussed with literature support. However, an experimental study conducted on the evaluation of TAC ocular tolerance on cattle cornea, no significant difference was reported between the group exposed to TAC and control group in terms of E-cadherin [29].
Hydroxyproline constitutes the main component of collagen. Hydroxyproline plays an important role in collagen stabilization. Hydroxyproline level is used as an indicator of collagen synthesis [30]. A study conducted on the effects of mTOR inhibitors on colon anastomosis recovery shows that mTOR inhibitors cause a decrease in tissue strength by decreasing protein synthesis. This finding was thought to be relevant with a decrease in hydroxyproline content. This finding also means a decrease in cell proliferation and angiogenesis. This means an increase in myeloperoxidase and matrix-metalloproteinase 2 and 9. As a result, proliferation phase is delayed. In our study, the level of hydroxyproline of TAC group was found to be significantly lower than other groups. Low level of hydroxyproline reflects a decrease in collagen synthesis. Decreasing collagen synthesis on the other hand may be the reason behind the decrease in tissue strength. Low bursting pressure of TAC group is consistent with decreasing hydroxyproline levels.
Whether preoperative application of calcineurin inhibitors increase in postoperative complications and its effects on colon anastomosis are still to be discussed [24].
In rats, the adverse effect of cyclosporin A has not been shown on primary wound healing [14]. In another study, anastomotic complications were less common in lung-transplanted dogs treated with cyclosporine [15]. On the other hand, Uzunkoy et al. [24] found adverse effects of CsA on intestinal anastomosis in rats. The effects of cyclosporine on colon anastamosis and wound healing are still controversial. There are limited studies and insufficient information on the efficiency of CsA to improve colon anastomosis. There was a significant decrease of bursting pressure in the CsA group compared to the control and isotonic groups; however, no significant difference in collagen deposition was observed. This may be related to the other important factor that affects anastomosis strength. CsA associated histopathologic changes at the ultrastructural level needs to be further analyzed. Therefore, further studies, such as electron microscopy for collagen cross-linking examination, are needed to clarify the pathophysiological events.
One of the study's limitations was that TAC and CsA can be created with different doses. Further, a single time point was used to evaluate colon anastomosis recovery in this study. Perhaps different time points could be considered in future studies. New studies are needed to evaluate the effects of different dosages in different time spans. The drug dosages used were based on previous studies [78]. However, monitoring immunosuppressive activity may be useful in assessing toxicity and efficiency of drugs used in future studies. CsA-treated rats had no significant differences in collagen, hydroxyproline, E-cadherin, despite bursting pressure being statistically lower in this group compared with the control and sham groups. This may be related to the other important factor that affects anastomosis strength, which is the cross-linking of collagen. Besides, mechanical strength of the anastomosis is also related to the structural collagen web in the submucosal layer of the colon. Thus, CsA-associated histopathologic changes at the ultrastructural level need to be further analyzed. Therefore, further studies are needed to clarify the pathophysiological events. Electron microscopy can be used to understand collagen cross-linking. The half-lives of TAC and CsA can vary greatly. Levels of these drugs can be checked for drug clearance.
In conclusion, our study showed that TAC might have a negative effect of colon anastomosis recovery. The lowest anastomosis bursting pressure was detected in TAC group. Further to this, collagen, hydroxyproline, fibroblast, neovascularization and E-cadherin levels were low in the TAC group. Even though the colon anastomosis bursting pressure significantly decreased in the CsA group, the decreased levels were more significant in the TAC group. Contrary to TAC group, CsA did not cause any significant change in tissue hydroxyproline, collagen, fibroblast, and E-cadherin levels.
Figures and Tables
 | Fig. 1Histopathologic findings between groups, E-Cadherin levels and anastomosis bursting pressure comparisons. (A) Fibroblast. P < 0.05, control vs . tacrolimus groups. (B) Neovascularization. P < 0.05, control vs. tacrolimus groups. (C) Collagen. P < 0.05, tacrolimus vs . other groups. (D) E-cadherin. P < 0.05, tacrolimus vs. other groups. (E) Bursting pressure of anastomosis (mmHg). P < 0.001, tacrolimus vs. other groups. P < 0.05, cyclos A vs. control and sham groups. |
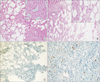 | Fig. 2Histopathologic evaluation of groups. (A) Control group: Dense inflammation, increase in fibroblast count, clarification of neovascularization, and a significant increase in collagen fibers (small photo) is observed (H&E, ×100). (B) Tacrolimus group: A decrease in inflammatory cells, fibroblast count, neovascularization, and collagen fiber formations (small photo) is observed (H&E, ×100). (C) Tacrolimus group: grade 0 coloring pattern (immunohistochemistry – E-cadherin, ×200). (D) Control group: grade 1 coloring pattern: examination conducted with E-cadherin (immunohistochemistry - E cadherin, ×200). |
ACKNOWLEDGEMENTS
The authors would like to thank Dicle University Department of Pathology for the pathological evaluation.
References
1. Willems MC, van der Vliet JA, Lomme RM, Hendriks T. Tacrolimus does not affect early wound healing in a rodent model of bowel anastomoses and abdominal wall closure. PLoS One. 2013; 8:e76348.
2. Seyhun Y, Ciftci HS, Kekik C, Karadeniz MS, Tefik T, Nane I, et al. Genetic association of interleukin-2, interleukin-4, interleukin-6, transforming growth factor-β, tumour necrosis factor-α and blood concentrations of calcineurin inhibitors in Turkish renal transplant patients. Int J Immunogenet. 2015; 42:147–160.
3. Chung BH, Kim KW, Kim BM, Piao SG, Lim SW, Choi BS, et al. Dysregulation of Th17 cells during the early post-transplant period in patients under calcineurin inhibitor based immunosuppression. PLoS One. 2012; 7:e42011.
4. Kita J, Ogino Y, Kobayashi E, Fujimura A, Kogure H. Effects of tacrolimus on small and large bowel anastomoses in the rat. Transplant Proc. 1999; 31:2789.
5. Willems MC, van der Vliet JA, de Man BM, van der Laak JA, Lomme RM, Hendriks T. Persistent effects of everolimus on strength of experimental wounds in intestine and fascia. Wound Repair Regen. 2010; 18:98–104.
6. Karaca G, Pekcici MR, Altunkaya C, Fidanci V, Kilinc A, Ozer H, et al. The effects of scalpel, harmonic scalpel and monopolar electrocautery on the healing of colonic anastomosis after colonic resection. Ann Surg Treat Res. 2016; 90:315–321.
7. Kedzierska K, Sindrewicz K, Sporniak-Tutak K, Bober J, Stanczyk-Dunaj M, Dolegowska B, et al. Effect of immunosuppressive therapy on proteinogram in rats. Med Sci Monit. 2016; 22:1987–1998.
8. Kabat-Koperska J, Kolasa-Wolosiuk A, Wojciuk B, Wojciechowska-Koszko I, Roszkowska P, Krasnodebska-Szponder B, et al. The influence of intrauterine exposure to immunosuppressive treatment on changes in the immune system in juvenile Wistar rats. Drug Des Devel Ther. 2016; 10:2279–2288.
9. Li Z, Sun F, Zhang Y, Chen H, He N, Chen H, et al. Tacrolimus induces insulin resistance and increases the glucose absorption in the jejunum: a potential mechanism of the diabetogenic effects. PLoS One. 2015; 10:e0143405.
10. Ehrlich HP, Tarver H, Hunt TK. Effects of vitamin A and glucocorticoids upon inflammation and collagen synthesis. Ann Surg. 1973; 177:222–227.
11. Tuncel H, Shimamoto F, Cagatay P, Kalkan MT. Variable E-cadherin expression in a MNU-induced colon tumor model in rats which exposed with 50 Hz frequency sinusoidal magnetic field. Tohoku J Exp Med. 2002; 198:245–249.
12. Pramateftakis MG, Kanellos D, Demetriades H, Kanellos I, Mantzoros I, Zacharakis E, et al. The effects of irinotecan on the healing of colonic anastomoses in rats. Open Surg J. 2007; 1:1–6.
13. Schaffer M, Fuchs N, Volker J, Schulz T, Kapischke M, Viebahn R. Differential effect of tacrolimus on dermal and intestinal wound healing. J Invest Surg. 2005; 18:71–79.
14. Eisinger DR, Sheil AG. A comparison of the effects of cyclosporin A and standard agents on primary wound healing in the rat. Surg Gynecol Obstet. 1985; 160:135–138.
15. Pinsker KL, Veith FJ, Kamholz SL, Emeson EE, Norin A, Montefusco C. Bronchial anastomotic healing in canine lung allotransplants treated with cyclosporine. Transplantation. 1985; 40:143–146.
16. Kuper MA, Trutschel S, Weinreich J, Konigsrainer A, Beckert S. Growth hormone abolishes the negative effects of everolimus on intestinal wound healing. World J Gastroenterol. 2016; 22:4321–4329.
17. Kanellos I, Mantzoros I, Demetriades H, Kalfadis S, Kelpis T, Sakkas L, et al. Healing of colon anastomoses covered with fibrin glue after immediate postoperative intraperitoneal administration of 5-fluorouracil. Dis Colon Rectum. 2004; 47:510–515.
18. Halsted W. Circular suture of the intestine an experimental study. Am J Med Sci. 1887; 94:436–461.
19. Savage FJ, Lacombe DL, Boulos PB, Hembry RM. Role of matrix metalloproteinases in healing of colonic anastomosis. Dis Colon Rectum. 1997; 40:962–970.
20. Pronio A, Di Filippo A, Narilli P, Mancini B, Caporilli D, Piroli S, et al. Anastomotic dehiscence in colorectal surgery. Analysis of 1290 patients. Chir Ital. 2007; 59:599–609.
21. Brunner G. Theme issue: Wound healing mechanisms. Thromb Haemost. 2003; 90:976–977.
22. Raptis D, Mantzoros I, Pramateftakis MG, Despoudi K, Zaraboukas T, Koliakos G, et al. The effects of tacrolimus on colonic anastomotic healing in rats. Int J Colorectal Dis. 2012; 27:299–308.
23. Turgut B, Guler M, Akpolat N, Demır T, Celıker U. The impact of tacrolimus on vascular endothelial growth factor in experimental corneal neovascularization. Curr Eye Res. 2011; 36:34–40.
24. Uzunkoy A, Akinci OF, Coskun A, Aslan O, Kocyigit A. Effects of antiadhesive agents on the healing of intestinal anastomosis. Dis Colon Rectum. 2000; 43:370–375.
25. Clark RA, Nielsen LD, Welch MP, McPherson JM. Collagen matrices attenuate the collagen-synthetic response of cultured fibroblasts to TGF-beta. J Cell Sci. 1995; 108(Pt 3):1251–1261.
26. Que J, Cao Q, Sui T, Du S, Kong D, Cao X. Effect of FK506 in reducing scar formation by inducing fibroblast apoptosis after sciatic nerve injury in rats. Cell Death Dis. 2013; 4:e526.
27. Janikowska G, Janikowsk T, Pyka A, Wilczok A, Mazurek U. Cyclosporin a affects the proliferation process in normal human dermal fibroblasts. Acta Pol Pharm. 2016; 73:55–63.
28. Cheong YC, Laird SM, Li TC, Shelton JB, Ledger WL, Cooke ID. Peritoneal healing and adhesion formation/reformation. Hum Reprod Update. 2001; 7:556–566.
29. Pastor-Clerigues A, Serrano A, Milara J, Marti-Bonmati E, Lopez-Perez FJ, Garcia-Montanes S, et al. Evaluation of the ocular tolerance of three tacrolimus topical pharmaceutical preparations by bovine corneal opacity and permeability test. Curr Eye Res. 2016; 41:890–896.
30. Cohen SR, Cornell CN, Collins MH, Sell JE, Blanc WA, Altman RP. Healing of ischemic colonic anastomoses in the rat: role of antibiotic preparation. Surgery. 1985; 97:443–446.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download