Abstract
Sentinel lymph node biopsy has been developed as the standard of treatment in breast cancer. Status of axillary sentinel lymph node is known to be a significant prognostic factor. Nevertheless, involvement of an intramammary lymph node with metastasis in breast cancer is a rare radiological and clinical presentation, and with extracapsular extravasation even more uncommon. Historically, reported series of patients with intramammary lymph node diagnosed by final histological examination are small in number and clinical significance of metastasis is still unclear. Here, we report a case of conservative breast cancer surgery with 3 intramammary sentinel lymph nodes containing metastasis and extracapsular extravasation. After multidisciplinary consensus, the patient was surgically reapproached with mastectomy. Even though the 3 intramammary sentinel lymph nodes were positive for metastases, pathology examination did not reveal any signs of malignancy in the mastectomy specimen.
Involvement of intramammary sentinel lymph node (IMSLN) with metastasis is a rare finding, and with extracapsular extravasation even more uncommon. Clinical significance, including prognosis and therapeutic approach of these IMSLNs is yet unclear and procedures are not defined. We report a case of conservative breast cancer surgery with 3 IMSLNs containing metastases and capsular extravasation. The patient was surgically reapproached with mastectomy; however, pathology examination did not reveal any signs of malignancy.
A 44-year-old, white postmenopausal female was referred to the Department of Breast Surgery of the AC Camargo Cancer Center (ACCCC) with a mammogram image of a partially defined lump in the lateral quadrants of the right breast, BI-RADS 0. Ultrasound of the breast showed a circumscribed hypo echoic nodule, with precise limits, 1.4 cm × 1.1 cm, disting 3.8 cm from the areolar complex and 1.3 cm from the skin, and also an adjacent hypoechoic microlobulated lesion of 1.4 cm × 1.2 cm × 1.4 cm in correspondence to the mammogram, both in the outer quadrants of the right breast, BI-RADS 4. Ultrasound-guided core-needle biopsy of the lesion revealed a high grade invasive ductal carcinoma of the right breast. The slides were formerly reviewed by the ACCCC Pathology department which confirmed malignancy and immunophenotype Luminal B (estrogen receptor 30%, progesterone receptor and human epidermal growth factor receptor 2 negative, and Ki-67 of 80%).
MRI of the breast for preoperative planning showed an oval mass with spiculated contours at early and heterogeneous postcontrast enhancement, located in the junction of the outer quadrants of the right breast, measuring 18 mm × 16 mm × 15 mm, 56 mm of the papilla, 22 mm of the skin and 31 mm from the pectoralis major muscle (Fig. 1A). In addition, a circumscribed oval mass at early and homogeneous postcontrast enhancement, and high signal intensity on T2, located in the posterior third of the lower inner quadrant of the right breast, measuring 9 mm × 5 mm × 5 mm and in close contact with the pectoralis major muscle, which seemed to correspond to an intramammary lymph node (IMLN) distant 95 mm of the papilla and 37 mm from the skin, was also identified (Fig. 1B). Therefore, a second-look ultrasound was performed directly to this lymph node, which revealed cortical thickening and a decreased hilum, considered suspicious. A breast conserving surgery of the index lesion, as well as radioguided occult lesion localization of the suspicious lesion in the lower inner quadrant of the right breast, in addition to axillary approach to investigate the sentinel lymph node (SLN), were performed. The final pathology report of the lesions identified an invasive carcinoma, no special type, histology grade III, nuclear grade 3 and associated ductal carcinoma in situ. Three axillary SLNs were free of metastases, however the suspected lesion located in the lower medial quadrant of the right breast resulted in 3 IMSLNs, all affected by cancer metastases (3/3) with capsular extravasation (Fig. 2).
Published literature for IMSLNs does not mention capsular leakage, thus, there is no consensus for the best treatment: reapproachment to radical surgery or only adjuvant therapy? The case was discussed at the Institution's tumor board. Pathologists admitted margins in lymph nodes are not frozen, which does not make it possible to know if the margins of the additional lesions were disease free. Tumor board recommendation was mastectomy without axillary lymph node dissection (ALND) and immediate breast reconstruction. The procedure was performed 2 weeks after patient's consent in regards to the unknown probability of further disease in the IMSLN. Pathology report of the mastectomy revealed chronic inflammatory process with foreign body giant cell reaction and bleeding areas, steatonecrosis, usual ductal hyperplasia, but no evidence of malignancy.
IMLNs have not received enough attention from the medical literature and many of their characteristics have not been sufficiently explored. They are clinically important because they can coexist as the primary sites of metastases and also as the SLNs. Frequently, IMLNs are mostly benign incidental findings, but when image identified, the presence of metastasis was more frequent than in IMLNs incidentally detected [1]. Nevertheless, literature data regarding the clinical significance of IMSLN metastases, remains controversial. Their radiological image at mammography is that of a well circumscribed dense mass with an area of lower density at the centre representing the hilum. At ultrasonography, usually the benign lymph node is of a circumscribed, hypoechoic mass with an echogenic hilum and thin cortical. Cortical thickening and absence of hilum are changes at high risk for metastases detected by ultrasonography with more specificity, according to many studies [234]. Therefore, ultrasonography is the most significant imaging method for lymph node evaluation [5]. Their role in lymphatic drainage of breast regions is important, although it is not known if they represent true sentinel nodes or if lymphatic drainage to them comes from ectopic, independently developed pathways. Their incidence in various studies ranges between 0.7% and 48% [67] and can be found in all breast quadrants. IMLNs are involved in a variety of clinical situations, including benign situations, tumor metastases, breast lymphomas, and breast cancer, where their importance is not fully established. Nevertheless, there is evidence that IMLNs are an independent factor for poor prognosis, and they may change therapeutic decisions. Patients with metastatic IMSLNs had more aggressive cancers with lymphatic and vascular invasion as well as increased axillary lymph node metastases [6]. The use of axillary SLN biopsy accurately represents the disease status of the axilla in cases of positive IMSLN, and the use of nomograms demonstrated that the risk of axillary metastasis was less than 10% [1]. When IMSLN metastases are identified in the breast specimens, results suggest that complete axillary lymph node dissection (CALND) may be based on the axillary SLN negative status and thus, CALND can be avoided in this setting [89]. Yet, among 7,140 patients, intramammary nodes were identified in 151 patients (2%), and axillary disease was verified in 61% of intramammary node-positive patients. No additional axillary disease was identified when ALND was performed in intramammary node-positive patients with negative axillary SLN biopsy results [1].
Even though our case has not demonstrated malignancy at the surgical site, capsular extravasation is considered a sign and risk of worse prognosis, but there is no evidence that leads us to conclude that IMSLNs metastases featuring capsular extravasation might be an independent outcome factor, and its clinical meaning is yet unknown. Hence, even surgical margins may be affected due to disease, as showed by Koca et al. [10]'s retrospective study, where authors found that positivity of SLNs significantly affected surgical margins. Therefore, further therapeutic approach is still undefined, and should be considered among other clinical features.
In conclusion, the current case has shown that an axillary SLN biopsy accurately represents the status of the axilla in cases with a positive IMSLN. ALND can be omitted in the setting of a positive IMSLN and a negative axillary SLN. However, capsular extravasation of the IMSLN was considered an aggressive feature and sign of worse prognosis, being thus important to consider reapproachment of the tumor site due to insufficient and unevaluated lymph node margins. Additional radiotherapy of the internal mammary chain drainage may also be considered.
Figures and Tables
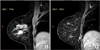 | Fig. 1(A) Postcontrast sagital T1-weighted magnetic resonance images of the right breast shows an irregular mass with spiculated margins and heterogeneous enhancement located in the junction of the outer quadrants, compatible with the primary tumor. (B) Small round mass with circumscribed margins and with homogeneous enhancement located in the posterior aspect of the lower inner quadrant, near the chest wall, which corresponded to a suspicious intramammary lymph node. |
References
1. Pugliese MS, Stempel MM, Cody HS 3rd, Morrow M, Gemignani ML. Surgical management of the axilla: do intramammary nodes matter? Am J Surg. 2009; 198:532–537.
2. Cho N, Moon WK, Han W, Park IA, Cho J, Noh DY. Preoperative sonographic classification of axillary lymph nodes in patients with breast cancer: node-to-node correlation with surgical histology and sentinel node biopsy results. AJR Am J Roentgenol. 2009; 193:1731–1737.
3. Mainiero MB. Regional lymph node staging in breast cancer: the increasing role of imaging and ultrasound-guided axillary lymph node fine needle aspiration. Radiol Clin North Am. 2010; 48:989–997.
4. Mainiero MB, Cinelli CM, Koelliker SL, Graves TA, Chung MA. Axillary ultra sound and fine-needle aspiration in the preoperative evaluation of the breast cancer patient: an algorithm based on tumor size and lymph node appearance. AJR Am J Roentgenol. 2010; 195:1261–1267.
5. Alvarez S, Anorbe E, Alcorta P, Lopez F, Alonso I, Cortes J. Role of sonography in the diagnosis of axillary lymph node metastases in breast cancer: a systematic review. AJR Am J Roentgenol. 2006; 186:1342–1348.
6. Lee SK, Kim S, Choi MY, Kim J, Lee J, Jung SP, et al. The clinical meaning of intramammary lymph nodes. Oncology. 2013; 84:1–5.
7. Abdullgaffar B, Gopal P, Abdulrahim M, Ghazi E, Mohamed E. The significance of intramammary lymph nodes in breast cancer: a systematic review and meta-analysis. Int J Surg Pathol. 2012; 20:555–563.
8. Vijan SS, Hamilton S, Chen B, Reynolds C, Boughey JC, Degnim AC. Intramammary lymph nodes: patterns of discovery and clinical significance. Surgery. 2009; 145:495–499.
9. Diaz R, Degnim AC, Boughey JC, Nassar A, Jakub JW. A positive intramammary lymph node does not mandate a complete axillary node dissection. Am J Surg. 2012; 203:151–155.
10. Koca B, Kuru B, Yuruker S, Gokgul B, Ozen N. Factors affecting surgical margin positivity in invasive ductal breast cancer patients who underwent breast-conserving surgery after preoperative core biopsy diagnosis. J Korean Surg Soc. 2013; 84:154–159.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download