Abstract
Purpose
To present the feasibility and safety of Roux-en-Y esophagojejunostomy using hemi-double-stapling technique after laparoscopic total gastrectomy.
Methods
We reviewed the outcomes from 58 consecutive patients with gastric cancer who underwent laparoscopic total gastrectomy. The clinicopathological characteristics including postoperative complications were examined.
Results
The mean age and body mass index were 57.3 ± 9.7 years and 23.7 ± 2.6 kg/m2, respectively. The mean overall total operation was 199.8 ± 57.0 minutes. Intraoperative blood loss was 81.6 ± 56.3 mL and there was no open conversion. The patients' hospital stay was a mean 9.6 ± 2 days. The mean proximal margin of the specimens was 2.7 ± 1.8 cm. There were 3 cases (5.1%) of anastomosis leakage, but all were controlled successfully by endoscopic stent.
Gastric cancer is the second most common cause of cancer death worldwide and the leading primary malignancy in Korea [12]. With the growing proportion of early gastric cancer (EGC) cases due to the widening use of effective cancer-screening policies, utilization of laparoscopic distal gastrectomy (LDG) as an EGC treatment option for Koreans has been increasing [34].
The reported advantages of laparoscopic gastrectomy compared with the open approach include less pain, earlier recovery, better quality of life, and improved cosmesis. Recently, the technical feasibility of LDG, not to mention long-term positive outcomes, has been proven [5]. Use of laparoscopic total gastrectomy (LTG) as a surgical option, meanwhile, is declining.
Since the first LTG for gastric cancer was performed by Huscher et al. [6] in 1992, there have been several reports of its feasibility and safety. Notwithstanding, LTG is regarded, still, as a difficult procedure that typically is performed only by experienced laparoscopic surgeons at relatively few hospitals and centers.
The important technical issue is the safety of lymph node dissection performed along the splenic hilum (No. 10), distal splenic artery (No. 11d), and short gastric arteries (No. 4sa). Moreover, esophagojejunostomy remains problematic due to the high rate of anastomosis failure including leakage or stenosis, though the introduction of newer stapling devices has alleviated that problem. One study reported rates of postoperative stricture at the esophagojejunostomy site of 9 and 1% in laparoscopic and open total gastrectomy, respectively [7]. Whereas various types of esophagojejunal anastomosis are presented in the literature, there is no widely accepted standard technique in LTG [8]. Over the years in which laparoscopic surgery has been performed, several reconstructive techniques of esophagojejunal anastomosis have been cited, such as extracorporeal esophagojejunostomy, circular stapler with the anvil placed transabdominally or transorally, or side-to-side anastomosis with linear stapler. In the case of the circular stapling technique, the purse-string sutures and anvil insertions are still problematic, due to the narrow surgical field of view or the need for more precise manipulations by more experienced physicians [910].
In recent years, we have performed more than 50 intracorporeal esophagojejunostomies using the hemi-double-stapling technique (HDST). On these pages, we discuss the method's feasibility and safety.
Between July 2010 and December 2014, 58 consecutive gastric cancer patients (37 men and 21 women) underwent LTG by the HDST at our hospital. Only clinical T1 gastric cancer patients were considered as candidates for LTG. Patient selection was based on preoperative examinations including esophagogastroduodenoscopy, endoscopic ultrasonography, and computed tomography. We reviewed and recorded the patients' characteristics as well as postoperative outcomes including operating time, time to 1st oral diet, hospitalization stay, and intraoperative blood loss. Additionally, reconstruction-related complications including anastomosis stricture, leakage and disruption were evaluated.
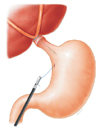
The patient was placed in the modified lithotomy position with the lower limbs extended and properly supported in the leggings proper. The surgeon stood on the patient's right side; the first assistant stood on the left, and the laparoscopic camera operator stood between the patient's legs. As in conventional laparoscopic gastrectomy, a total of 5 abdominal ports were established: three 12-mm ports and two 5-mm ports. The first, a 12-mm umbilical port, was established for insertion, by the open technique, of a CO2 pneumoperitoneum, its pressure maintained at 12–14 mmHg. The second 12-mm port was employed as the main operating port on the right anterior axillary line below the costal margin. The third 12-mm port, established 5 cm to the left side of the umbilicus mid-clavicular line, was used as the assistant operating port. Finally, the two 5-mm ports were established 5 cm below the costal margin on the right and left, respectively. After completing the resection procedure including D1+ lymph node dissection (except for the esophageal transection), Rouxen-Y reconstruction was performed by modified hemi-double-stapling esophagojejunostomy, as follows. First, a 3- to 4-cm mini-laparotomy was performed at the site of the assistant 12-mm port in the left midaxillary line. The mini-laparotomy incision was retracted and protected with a laparotomy wound retractor (Alexis Wound Retractor; Applied Medical, Rancho Santa Margarita, CA, USA). Through this mini-laparotomy, the anvil (Premium plus CEEA 25, US Surgical, Norwalk, CT, USA) was inserted into the abdominal cavity, and the pneumoperitoneum was reestablished by sealing off the laparotomy wound retractor with a surgical glove. Second, the Roux-en-Y esophagojejunostomy was performed by gastrotomy approximately 1 to 2 cm below the esophagogastric junction (away from the tumor mass), and could be extended approximately 3 cm across the esophagogastric junction using a harmonic scalpel (Ethicon Endo-Surgery, Cincinnati, OH, USA). The prepared circular stapler anvil, preferably number 25, was inserted into the esophagus through the gastrotomy incision and then positioned in the thoracic esophagus (Fig. 1). To reduce the risk of spillage, we used the umbilical tape to pull the stomach and also we aspirated the gastric fluid right after making the gastrotomy.
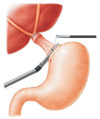
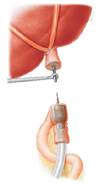
Subsequently, the distal esophagus was transected with an Endo GIA linear stapler (Endo GIA 60, US Surgical, Norwalk, CT, USA) with a disposable GI blue cartridge (Fig. 2), after which the thread attached to the anvil was pulled farther outside until the rod of the anvil was completely outside of the anterior wall of the esophagus. First, end-to-side hand sewing jejunojejunal anastomosis was performed extracorporeally. Then, the circular stapler was inserted into the jejunum and temporarily fixed with rubber bands. The anastomotic device and small intestine were positioned in the abdomen, and a pneumoperitoneum was reestablished by clipping the abdominal wall with towel forceps. Subsequently, end-to-side esophagojejunal anastomosis was performed under laparoscopy (Fig. 3), and the jejunal stump was closed using the linear stapler. Digestive tract reconstruction was performed, and a drainage tube was positioned beside the anastomotic stoma. Finally, to avoid disrupting the stapler line, pursing interrupted suture was intracorporeally applied, and the resected specimen was pulled out through the mini-laparotomy incision.
The mean age and body mass index were 57.3 ± 9.7 years and 23.7 ± 2.6 kg/m2, respectively (Table 1). The mean overall total operation time including reconstruction and anvil insertion was 199.8 ± 57.0 minutes. Intraoperative blood loss was 81.6 ± 56.3 mL, and there was no open conversion. Splenectomy was necessary in 2 patients (3.4%). The patients' hospital stay was a mean 9.6 ± 2 days. The mean proximal margin of the specimens was 2.7 ± 1.8 cm (Table 2). The postoperative complications were categorized according to the EJ anastomosis and non-EJ anastomosis sites. At the EJ site, there were 3 leak cases (5.1%), all of which were controlled successfully by endoscopic stent; there were 5 cases (8.6%) of anastomosis stricture, which were treated successfully by endoscopic intervention, except for 1 case (1.7%) that required surgical intervention. There were 3 cases (5.1%) of A-loop partial obstruction, which were treated conservatively, and a single case (1.7%) of A-loop bleeding was treated conservatively as well. As for the non-EJ site complications, the majority were chest-related: 3 pneumonia cases (5.1%), 4 pleural effusion (6.8%), 2 pancreatitis (3.4%), and 1 splenic bleeding (1.7%), which necessitated a splenectomy to control the hemorrhage. Additionally, there was intra-abdominal fluid collection was in 6 cases (10.3%), wound infection in 2 cases (3.4%), wound disruption in 1 case (1.7%), ileus in 5 cases (8.6%), and trocar site hematoma in 1 case (1.7%).
Despite the significant growth in the use of laparoscopic surgery, many surgeons have been hesitant to perform LTG, due to its technical difficulties, particularly the difficulties with anastomosis relative to lymph node dissection [11]. A simple and secure reconstructive technique is necessary to facilitate the use of LTG for treatment of upper gastric cancer. Esophagojejunostomy techniques for example, in order to reach their full potential, require simplification to enable more surgeons to perform them. The extracorporeal use of circular staplers has been preferred over their intracorporeal use, because it is difficult to wield them under the limited laparoscopic field of view.
In recent years, various types of intracorporeal anastomosis techniques have been developed that simplify esophagojejunostomy [8]. However, there is still debate about the optimal reconstruction procedure. Intracorporeal reconstruction can be largely divided into end-to-side anastomosis using a circular stapler and side-to-side anastomosis using a linear stapler. Side-to-side esophagojejunal anastomosis is designed to reduce the complexity of circular-staple anastomosis by obviating the need for purse-string sutures [12]. Also, it is relatively easy to perform under the limited laparoscopic field of view, thereby reducing the risk of injury to the esophageal wall as compared with the circular stapler. Moreover, with side-to-side esophagojejunal anastomosis, mini-laparotomy for esophagojejunostomy is not necessary. However, in cases of patients with a short abdominal esophagus, even side-to-side anastomosis using a linear stapler is a complex and difficult procedure, though some length for anastomosis could be obtained through crural dissection [13].
Laparoscopic techniques employing the circular stapler require an experienced surgeon and a skilled assistant. The key to this anastomosis is performing the purse-string suture and inserting the anvil into a fragile/contracted esophagus. Some expert surgeons can perform manual intracorporeal purse-string suturing, which serves simplicity and safety. The development of laparoscopic instruments such as the Endostich (Auto suture, Tyco Healthcare, Mansfield, MA, USA) or Endo-PSI (II) (Hope Electronics, Chiba, Japan), moreover, has reduced the difficulty of laparoscopy. Nonetheless, precise purse-string suturing and anvil insertion remain challenging.
The HDST can be both a useful and a time-saving procedure given the simplicity of performing the purse-string suture with it. This procedure, significantly, makes anvil insertion possible without intracorporeal suture. Also, since only the anterior wall of the stomach is incised to form the anvil entry hole prior to transection of the esophagus, tearing of the esophageal muscle fiber and mucosa, which is a critical factor in anastomotic complications, can be prevented. We believe that the HDST, therefore, can be a very effective option, especially for inexperienced surgeons. However, there is the concern about tumor spillage during hemi-double-stapling technique, because we should open the stomach during operation. To reduce the risk of spillage, we used the umbilical tape to pull the stomach and also we aspirated the gastric fluid right after making the gastrotomy. Although several cases in this series were proven as advanced cases, we applied this technique only for clinical T1 case. We looked at the long-term outcomes for the cases and fortunately there is no patient with recurrence. But we believe that the long-term surveillance should be needed.
The rate of leakage or stricture is higher than the historical data. Most of the leakage cases were occurred in the beginning series although the stricture is still problematic. We previously looked at the reported series and found similar outcomes [81214151617]. Most of the leakage can be overcome with accumulation of experience; however, the rate of stricture would be higher than open cases even after learning curve. We believe that more sophisticated method should be developed in this point.
In summary, the circular HDST technique is simple and reliable without any significant demerits with respect to safety concerns or difficulty of operation.
Figures and Tables
Fig. 1
The prepared anvil of the circular stapler was inserted into the esophagus through the gastrotomy incision and then positioned in the thoracic esophagus.
References
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005; 55:74–108.
2. Strong VE, Song KY, Park CH, Jacks LM, Gonen M, Shah M, et al. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg. 2010; 251:640–646.
3. Song KY, Kim SN, Park CH. Laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for gastric cancer: technical and oncologic aspects. Surg Endosc. 2008; 22:655–659.
4. Kodera Y, Fujiwara M, Ohashi N, Nakayama G, Koike M, Morita S, et al. Laparoscopic surgery for gastric cancer: a collective review with meta-analysis of randomized trials. J Am Coll Surg. 2010; 211:677–686.
5. Kim HH, Han SU, Kim MC, Hyung WJ, Kim W, Lee HJ, et al. Long-term results of laparoscopic gastrectomy for gastric cancer: a large-scale case-control and case-matched Korean multicenter study. J Clin Oncol. 2014; 32:627–633.
6. Huscher CG, Chiodini S, Recher A. Laparoscopy-assisted gastrectomy for cancer: initial experience. In : 1st International Gastric Cancer Congress; 1995 Mar 29-Apr 1; Kyoto, Japan. Bologna (IT): Monduzzi editore, International Proceedings Division;1995. p. 1215–1219.
7. Hanisch E, Ziogas D, Baltogiannis G, Katsios C. Laparoscopic total gastrectomy: further progress in gastric cancer. Surg Endosc. 2010; 24:2355–2357.
8. Shim JH, Yoo HM, Oh SI, Nam MJ, Jeon HM, Park CH, et al. Various types of intracorporeal esophagojejunostomy after laparoscopic total gastrectomy for gastric cancer. Gastric Cancer. 2013; 16:420–427.
9. Salih AE, Bass GA, D’Cruz Y, Brennan RP, Smolarek S, Arumugasamy M, et al. Extending the reach of stapled anastomosis with a prepared OrVil™ device in laparoscopic oesophageal and gastric cancer surgery. Surg Endosc. 2015; 29:961–971.
10. Inokuchi M, Otsuki S, Fujimori Y, Sato Y, Nakagawa M, Kojima K. Systematic review of anastomotic complications of esophagojejunostomy after laparoscopic total gastrectomy. World J Gastroenterol. 2015; 21:9656–9665.
11. Jeong O, Park YK. Clinicopathological features and surgical treatment of gastric cancer in South Korea: the results of 2009 nationwide survey on surgically treated gastric cancer patients. J Gastric Cancer. 2011; 11:69–77.
12. Inaba K, Satoh S, Ishida Y, Taniguchi K, Isogaki J, Kanaya S, et al. Overlap method: novel intracorporeal esophagojejunostomy after laparoscopic total gastrectomy. J Am Coll Surg. 2010; 211:e25–e29.
13. Shim JH, Oh SI, Yoo HM, Jeon HM, Park CH, Song KY. Short-term outcomes of laparoscopic versus open total gastrectomy: a matched-cohort study. Am J Surg. 2013; 206:346–351.
14. Takiguchi S, Sekimoto M, Fujiwara Y, Miyata H, Yasuda T, Doki Y, et al. A simple technique for performing laparoscopic purse-string suturing during circular stapling anastomosis. Surg Today. 2005; 35:896–899.
15. Usui S, Nagai K, Hiranuma S, Takiguchi N, Matsumoto A, Sanada K. Laparoscopy-assisted esophagoenteral anastomosis using endoscopic purse-string suture instrument “Endo-PSI (II)” and circular stapler. Gastric Cancer. 2008; 11:233–237.
16. Hiki N, Fukunaga T, Yamaguchi T, Nunobe S, Tokunaga M, Ohyama S, et al. Laparoscopic esophagogastric circular stapled anastomosis: a modified technique to protect the esophagus. Gastric Cancer. 2007; 10:181–186.
17. Lee SE, Ryu KW, Nam BH, Lee JH, Kim YW, Yu JS, et al. Technical feasibility and safety of laparoscopy-assisted total gastrectomy in gastric cancer: a comparative study with laparoscopy-assisted distal gastrectomy. J Surg Oncol. 2009; 100:392–395.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download