Abstract
Transplantation of the horseshoe kidney can be performed en bloc or split into 2 grafts according to the vascular anomaly and the existence of the urinary collecting system in isthmus. From 2011 to 2014, there were 3 horseshoe kidney transplantations in Korea and transplantations were performed at 2 different centers. The transplantations were carried out successfully for all recipients without complications. All recipients have shown good graft kidney function after transplantation. No severe complication was revealed during follow-up period. We described the surgical technique used in the en bloc method to overcome various vascular anomalies and difficulties in choosing cannulation site and postoperative complications. En bloc transplantation of a horseshoe kidney is a useful strategy for patients with end-stage renal disease, and can provide favorable outcomes compared to the transplantation of a normal kidney.
Kidney transplantation is the most desired and cost-effective modality of renal replacement therapy for patients with endstage renal disease (ESRD). Since the implementation of the Korean Organ Transplant Act regarding brain death in February 2000, the number of patients waiting for kidney transplantation has been increasing dramatically. However, the progressive shortage of available donor kidneys has limited its clinical application and become a major barrier nationwide. In spite of utmost efforts to increase available donated kidneys from brain-dead donors, the discrepancy between suitable kidneys for transplantation and patients awaiting transplantation has been widening.
Because of the organ shortage, kidneys with atypical anatomy are frequently considered for transplantation. Horseshoe kidney is one of the most common anatomical variations of kidneys with an estimated incidence of 1 in 600–800 [1]. The anomaly of horseshoe kidney's anatomy results from anomalous fusion, mainly at the lower poles, occurring between the 4th and 6th weeks of gestation and its incidence is twice as often in men [2]. A horseshoe kidney can be transplanted en bloc or it can be divided and implanted into 2 recipients, depending on the anatomy of the vessels and the urinary collecting system [3]. Since Nelson and Palmer [4] reported the first case of transplanting horseshoe kidney, about 55 horseshoe kidney transplantation cases have been reported worldwide. Of these cases, 15 were transplanted en bloc [5]. Recently, the case of a horseshoe kidney divided from a live donor and successfully transplanted into a recipient has been reported [6]. When transplanting the horseshoe kidney, several factors such as kidney size, vascular anomalies and urinary tract anatomy should be considered. Vascular anomaly is the most frequent encounter for transplantation, and only 30% of all horseshoe kidneys have a single renal artery to each side. The thickness of the lower pole fusion, named the isthmus, also varies. The majority of horseshoe kidneys have a relatively thick isthmus consisting of renal parenchyma and urinary collecting system [2]. These features make it more difficult to transplant a horseshoe kidney. Therefore, individualized strategies of transplanting a horseshoe kidney are necessary. Since the Korean Network for Organ Sharing was established in 2000, there have been 3 cases of horseshoe kidney en bloc transplanted from brain-dead donors in Korea.
The first deceased donor, a 43-year-old male with a history of hypertension and chronic alcohol intake, was declared brain dead secondary to an intracranial hemorrhage. Informed consent for organ donation was taken from his family. Other medical or social history such as diabetes, viral hepatitis, urinary tract infection, and drug consumption was denied. His renal function was well preserved. The initial serum creatinine level was 1.2 mg/dL and estimated glomerular filtration rate (eGFR) was 70 mL/min/1.73 m2 and urine output was 100–200 mL/hr without proteinuria. On his CT scan before the organ procurement surgery, hepatosplenomegaly, gastric varices, and horseshoe kidney were found (Fig. 1). Multiple renal arteries supplying the horseshoe kidney from the aorta of donor were identified, and the isthmus of horseshoe kidney consisted of a normal renal parenchyma. Two ureters were seen bilaterally. Because of multiple renal arteries and a relatively thick isthmus, which might include a complex urinary collecting system and intrarenal vascular system, we made the decision to transplant the horseshoe kidney in an en bloc fashion. The horseshoe kidney was procured including the abdominal aorta, inferior vena cava (IVC), and 2 ureters.
The second donor, a 59-year-old male with hypertension and diabetes was declared brain dead secondary to cerebral infarction. He did not have hypertension, diabetes, and hepatitis, and his initial serum creatinine level was 2.33 mg/dL. Horseshoe kidney was not detected on ultrasonography before procurement. During the organ procurement surgery, the unique feature of the donor's horseshoe kidney was revealed and the kidney was procured including the abdominal aorta, IVC, and 2 ureters.
The third donor, 68-year-old female without any previous medical history was declared brain dead secondary to intracranial hemorrhage. She did not have any underlying disease such as hypertension, diabetes, or hepatitis. Her kidney function was well preserved. She was not supported by hemodialysis and her serum creatinine level was 0.6 mg. Her daily urine output was maintained slightly over 2,000 mL. The horseshoe kidney was revealed on ultrasonography before procurement. The kidney was procured en bloc with the abdominal aorta, IVC, and 2 ureters.
After the organ procurement surgery, bench procedure was done. The horseshoe kidney had a relatively thick isthmus consisting of renal parenchyma sized approximately 1.8 cm. The vascular anatomy consisted of 5 renal arteries all coming from the donor's abdominal aorta with each renal vein draining into the IVC (Fig. 2A). The ureters and collecting systems bilaterally ran through the anterior surface of the horseshoe kidney. Each time-zero biopsy from the upper poles of the horseshoe kidney for frozen section, revealed no histologic abnormalities. The second horseshoe kidney was large in size, 25 cm × 25 cm. Two ureters were seen bilaterally and the left ureter showed hydronephrotic change. Size of the third kidney was not measured. The horseshoe kidney had 2 renal arteries arising from abdominal aorta on each side and 1 renal vein draining into the IVC on each side (Fig. 2B). Two ureters showed normal features.
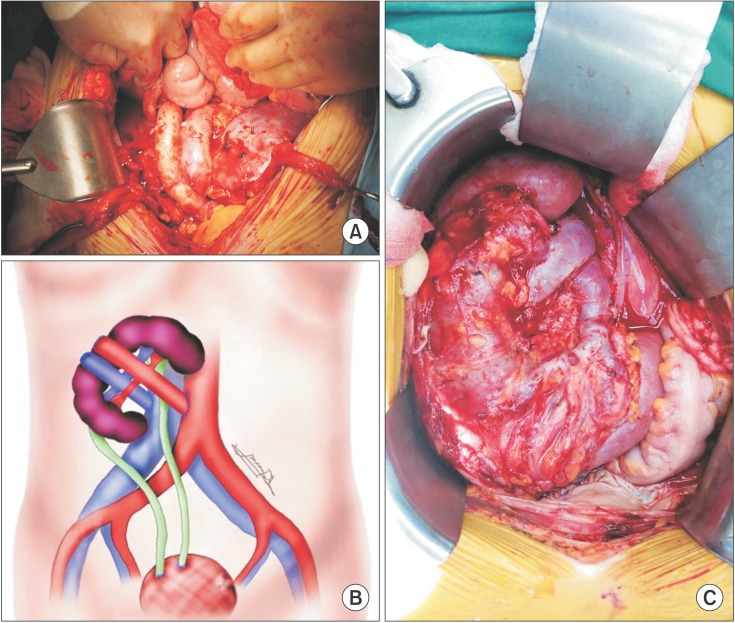
For the first recipient, because of insufficient space for transplanting a horseshoe kidney in the retroperitoneal space, we decided to make a midline skin incision in order to prepare a sufficient intraperitoneal space for the big horseshoe kidney, rather than a conventional retro-peritoneal iliac fossa. The whole segment including 5 renal arteries of the donor's aorta and renal veins of the donor's vena cava were used to their vascular anastomoses. The proximal ends of the aorta and the vena cava were closed with continuous running sutures. The distal ends of the aorta and the vena cava were anastomosed to the recipient's right common iliac artery and right common iliac vein in end-to-side fashions, respectively (Fig. 3A). Immediately after the reperfusion, the horseshoe kidney turned a normal color, and made a substantial amount of urine. The ureters were joined to each other in the double-barrel fashion, and double-J stents were placed in both ureters. The joined ureters of the horseshoe kidney were implanted into the bladder by an extravesical method using the antireflux technique according to Lich-Gregoir technique. The cold ischemic time was 6 hours, and the warm ischemic time was 58 minutes.
In the second recipient, the kidney was also transplanted in the intraperitoneal space. Due to the large size of the horseshoe kidney, we decided to place the kidney flipped and upside down. (Fig. 3B) Ascending colon and cecum were mobilized to expose aorta and IVC. The kidney was placed on the right lower quadrant of the abdomen. Donor's aorta and IVC were anastomosed to the recipient's aorta and IVC in the end-to-side fashion with prolene 4-0. Both ureters were implanted to the bladder separately by using Lich-Gregoir technique. Recipient's bladder capacity was 500 mL and double-J stent was not inserted. The cold ischemic time was 6 hours, and the warm ischemic time 95 minutes.
In the third recipient, the kidney was also transplanted into the intraperitoneal space. The kidney was placed on the right side of the abdomen to make graft renal arteries and veins perpendicular to the recipient's aorta and IVC. The distal ends of the aorta and IVC were closed by continuous sutures. The proximal ends of the aorta and IVC were anastomosed to the recipient's aorta and IVC in the end-to-side fashion with prolene 4-0 and prolene 6-0, respectively. Both ureters were anastomosed to the bladder seperately by using Lich-Gregoir technique. Due to the recipient's bladder capacity of 140 mL, double-J stents were inserted in each ureter (Fig. 3C). The cold ischemic time was 5 hours and the warm ischemic time was 58 minutes.
The graft of the first recipient functioned immediately after reperfusion. However, the urine output was poor at about 500 mL/day and serum creatinine was 6.9 mg/dL on the first day of transplantation. The recipient's serum creatinine level was increased up to 8.2 mg/dL until posttransplant 3 days. In spite of high serum creatinine, urine output had been steadily increasing, and eventually serum creatinine levels started declining from 4 days posttransplantation. The hemodialysis was not required after transplantation. On the Doppler ultrasonography at 5 days after transplantation, the implanted horseshoe kidney revealed normal blood flow without any vascular thrombosis or any perfusion defect. On the radioisotope renal scintigraphy using technetium-99m diethylenetriaminepentaacetic acid (99mTcDTPA) at 6 days posttransplantation, excretion of 99mTcDTPA through the horseshoe kidney was minimally delayed without any evidence of urinary leakage or obstruction. She was home on day 10 posttransplantation, and her serum creatinine level was 2.2 mg/dL and eGFR was 25 mL/min/1.73 m2 at that time. At 3 weeks after the operation, the serum creatinine level was 1.1 mg/dL and the surgical wound was completely healed without any evidence of surgical complications. At one month posttransplant, the recipient's 24-hour urine was collected at 2,100 mL and showed 682.5 mg of creatinine excretion, 970.2 mg of proteinuria, 45.2 mL/min/1.73 m2 of creatinine clearance. At 6 months posttransplant, the recipient's 24-hour urine was collected at 2,800 mL and showed 1,268.4 mg of creatinine excretion, no proteinuria, 89.7 mL/min/1.73 m2 of creatinine clearance. During the follow-up period until 19 months posttransplantation, there were no complications or rejection episodes.
The second graft kidney also functioned immediately after reperfusion. The urine output of the first postoperative day was 6,665 mL and serum creatinine levels declined from 14.24 mg/dL to 8.7 mg/dL. The Doppler ultrasonography on the first, 4th and 9th postoperative day showed patent vasculatures. The radioisotope renal scintigraphy using 99mTcDTPA on the 6th postoperative day revealed minimal delayed excretion in the upper moiety of the transplanted kidney without significant structural obstruction or leakage. He was discharged on 27th postoperative day with a serum creatinine level of 1.05 mg/dL and with no surgical complication such as wound problem or urinary leakage. At 2-month posttransplant, his serum creatinine level went up to 1.63 mg/dL. Kidney biopsy was done and revealed acute cellular rejection. He was treated with a steroid pulse therapy and discharged with a serum creatinine level of 1.12 mg/dL. At 3-month posttransplant, serum creatinine levels went up again to 1.66 mg/dL. Kidney biopsy was done and proved polyoma BK virus nephritis. Leflunomide use was started and mycophenolic acid was discontinued. Leflunomide had been used until polyoma BK virus was not detected in serum for 6 months. Since these episodes, his kidney function has been well maintained for 3 years without vasculature and urinary complications related with unusual anatomical structure. Posttransplantation follow-up duration was 39 months.
The third kidney began making a large amount of urine right after reperfusion. The urine output of the first postoperative day was 13,234 mL and serum creatinine levels declined from 13.3 mg/dL to 3.18 mg/dL. The urine output of the second postoperative day was 11,960 mL and serum creatinine levels declined to 0.84 mg/dL. Doppler ultrasonography at 1st, 3rd, 5th postoperative day confirmed patent renal vasculature. The radioisotope renal scintigraphy using 99mTcDTPA on the 5th postoperative day showed that the kidney had normal excretion function without anatomical obstruction or leakage. She used desmopressin nasal spray due to excessive daily urine output of more than 10,000 mL for 5 days. Except polyuria, she did not experience any unexpected events before discharge. She was discharged on the 21st postoperative day with serum creatinine levels at 0.68 mg/dL. Posttransplantation follow-up duration was 2 months and her kidney function was well maintained during this period.
Improvement in the medical management of progressive renal insufficiency and ESRD have led to growing numbers of potential transplant recipients despite a narrow donor pool. Under the circumstance, the effort to extend the donor pool is very important to meet organ demand. The horseshoe kidney, which is the most common renal anomaly, should be considered as a potential resource for kidney transplantation.
There are 2 surgical methods in horseshoe kidney transplantation: en bloc transplantation into a recipient and transplantations into 2 recipients after splitting of the horseshoe kidney. Because the vascular anatomy of the horseshoe kidney is commonly complex with more than a single renal artery on each side, many surgeons prefer to perform the transplantation en bloc. The most important factor in choosing the type of horseshoe kidney is how thick the parenchymal isthmus is. If the renal isthmus is thick and/or fibrotic, there is a higher risk of injury to the urinary collecting system during separation. Splitting the thick isthmus of the horseshoe kidney can lead to urinary fistula or to postoperative bleeding [7]. On the contrary, Stroosma et al. [8] reported that there were no significant differences of short and long-term posttransplant outcomes between the 2 methods. Nevertheless, many factors should be considered before making the decision to transplant en bloc or to split the horseshoe kidney.
Uzzo et al. [9] proposed an algorithm for evaluation and utilization of horseshoe kidneys for cadaveric transplantation. In the algorithm, renovascular anatomy, degree of fusion, ureteral anatomy and length were anatomic assessment points in choosing transplant method. For example, horseshoe kidney with multiple vessels, thick isthmus, and no disparity in ureteral length should be transplanted in the en bloc fashion. In the first case, the donor's horseshoe kidney had 5 arteries, a relatively thick isthmus, and sufficient ureter length. Because 2 of the 5 arteries supplied the isthmus, the part of the horseshoe kidney should be inevitably ischemic while splitting the isthmic portion. A previous report demonstrated that urinary leakage and fistula happened after splitting and implanting a horseshoe kidney with an 8-mm-thick isthmus [10]. Only when the urinary collecting systems of each side are apart from the isthmus, the isthmus can be safely divided. In the first case, we did not specifically evaluate the urinary collecting system, because we already chose en bloc transplantation on the basis of predonation contrast CT scan, which demonstrated that the isthmus was thicker than 1 cm and was supplied by 2 arteries.
Horseshoe kidney is frequently associated with vascular anomaly, in which renal arteries come from the lower part of the aorta or even from the common iliac artery. In order to perfuse the preservation solution through the whole part of the horseshoe kidney, we put a cannula into the donor's right common iliac artery rather than into the aorta. If the horseshoe kidney can be split at the isthmus, the site of cannulation could be the lower part of the aorta as usual after splitting the isthmus by using special cutting devices. However, when en bloc transplantation of the horseshoe kidney is chosen, careful selection of the site of cannulation is necessary and the iliac artery would be best.
A horseshoe kidney is one of the most valuable resources for kidney transplantation. It may be underutilized unless careful evaluation of the vascular, urinary, and parenchymal structures of the horseshoe kidney, proper choice of surgery plan, meticulous procurement and implantation, intensive preoperative and postoperative care can accompany. En bloc transplantation of a horseshoe kidney is one of the most useful strategies for patients with ESRD, and can provide a favorable outcome compared to the transplantation of a normal kidney.
References
1. Zipitis CS, Augustine T, Tavakoli A, Surange R, Agrawal A, Riad HN. Horseshoe kidney transplantation. Surgeon. 2003; 1:160–163. PMID: 15570753.

2. Stroosma OB, Scheltinga MR, Stubenitsky BM, Kootstra G. Horseshoe kidney transplantation: an overview. Clin Transplant. 2000; 14:515–519. PMID: 11127302.

3. Stroosma OB, Schurink GW, Smits JM, Kootstra G. Transplanting horseshoe kidneys: a worldwide survey. J Urol. 2001; 166:2039–2042. PMID: 11696702.

4. Nelson RP, Palmer JM. Use of horseshoe kidney in renal transplantation: technical aspects. Urology. 1975; 6:357–359. PMID: 1099770.

5. Pontinen T, Khanmoradi K, Kumar A, Kudsi H, Cheng Kung S, Chewaproug D, et al. Horseshoe kidneys: an underutilized resource in kidney transplant. Exp Clin Transplant. 2010; 8:74–78. PMID: 20199375.
6. Huser N, Geraurer KE, Novotny AR, Assfalg V, Stangl MJ. Successful living donor transplantation of a kidney with horseshoe malformation: extending the donor pool. Transpl Int. 2005; 18:761–762. PMID: 15910309.

7. Hau HM, Morgul HM, Uhlmann D, Thelen A, Fellmer P, Benckert C, et al. Horseshoe kidney for transplantation: technical considerations. Scand J Urol. 2013; 47:76–79. PMID: 22835080.

8. Stroosma OB, Smits JM, Schurink GW, de Boer J, Persijn GG, Kootstra G. Horseshoe kidney transplantation within the eurotransplant region: a case control study. Transplantation. 2001; 72:1930–1933. PMID: 11773891.
9. Uzzo RG, Hsu TH, Goldfarb DA, Taylor RJ, Novick AC, Gill IS. Strategies for transplantation of cadaveric kidneys with congenital fusion anomalies. J Urol. 2001; 165:761–765. PMID: 11176462.

10. Foster JT, Morrissey PE. Segmental renal ischemia following transplantation of horseshoe kidney as separate allografts. Case Rep Transplant. 2013; 2013:852127. PMID: 23476879.

Fig. 1
Deceased donor's CT scan. Image shows isthmus of horseshoe kidney consisting of renal parenchyma.
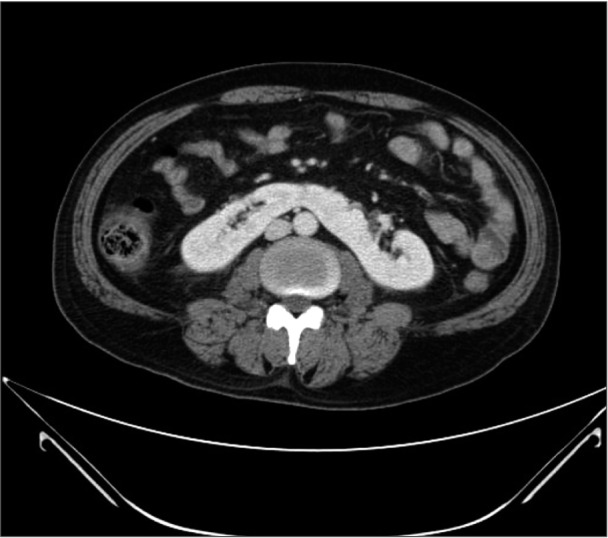
Fig. 2
(A) During the first donor's bench procedure, horseshoe kidney showed 5 arteries (white arrows) and 2 veins (white arrows) drained to graft aorta and vena cava. (B) In the third case, 2 arteries and 1 vein on each side were shown at back-table procedure.
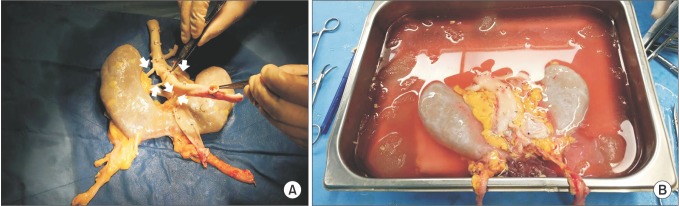
Fig. 3
Photograghed and drawn images of each horseshoe kidney after all anastomosis was done. (A) Graft aorta and vena cava were seen in middle of graft kidney. (B) Graft kidney placed upside down because lower portion of kidney was too large to be put into pelvic cavity. (C) Graft kidney was placed on right side of abdominal cavity to make graft vasculature perpendicular to recipient's aorta and inferior vena cava.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download