Abstract
Encapsulating peritoneal sclerosis (EPS) is a rare cause of intestinal obstruction by a thick fibrous membrane wrapping around the small intestine. It is a possible complication after liver transplantation (LT) that can be fatal. This report describes 2 cases of EPS after LT that were successfully treated with surgery, corticosteroids, tamoxifen, and mammalian target of rapamycin inhibitor. After treatment in both cases, the patients were able to start oral feeding and have been symptom free for more than 1 year. These cases suggests that for the management of EPS, surgical treatment is mandatory when the patients present with symptoms of intestinal obstruction or if there are findings suggestive of decreased mural perfusion. Surgery should be accompanied with medical treatment to prevent the relapse of EPS.
Encapsulating peritoneal sclerosis (EPS) is defined as ‘a clinical syndrome with persistent, intermittent and recurrent presence of intestinal obstruction with or without the existence of inflammation parameters and the existence of peritoneal thickening, sclerosis, calcifications and encapsulation confirmed by macroscopic inspection or radiological findings’ and is a rare but severe complication most often related to peritoneal dialysis (PD) [1]. The reported incidence of EPS is 0.5%–2.5% in patients treated with PD [2] and the incidence of EPS after kidney transplantation has increased [3]. The pathogenesis of this condition remains unclear, however, the universal use of calcineurin inhibitors (CNIs) is one of the proposed explanations for this increased incidence, as CNI has pro-fibrotic effects that may promotes EPS [4]. Cases of EPS after liver transplantation (LT) have also been reported [56], and its pathogenesis has been suggested to be due to recurrent peritoneal infection and irritation. However, the treatment strategy for EPS has not yet been established, especially in LT recipients. Here, we report 2 cases of LT recipients developed EPS after LT and were successfully treated with surgery and medications, including corticosteroids, tamoxifen, and mammalian target of rapamycin (mTOR) inhibitor.
Case 1 was a 55-year-old man who suffered from alcoholic liver cirrhosis (LC) without other significant past medical or surgical histories. He was diagnosed with LC in 2001 and had 3 documented cases of spontaneous bacterial peritonitis (SBP). He underwent deceased donor liver transplantation due to uncontrolled ascites and hepatic encephalopathy in October, 2003. Fibrotic changes of the peritoneum were not observed during the operation. He underwent emergency operation for bleeding control after LT on postoperative day 3. His maintenance immunosuppressive agents were tacrolimus (FK), mycophenolic acid (MPA), and prednisone. His general condition continued to be stable. He was admitted for cholangitis 2 times in 2012 and treated with antibiotics without any interventions. He was admitted with abdominal pain, nausea, and vomiting 11 years after LT. His WBC count was within normal range (5.41 × 103/µL) and CRP level was increased (2.54 mg/dL). He complained of abdominal pain and distention, but rebound tenderness was not detected. The abdominal X-ray was not suggestive of mechanical obstruction. Contrast abdominal CT revealed a large amount of ascites and the small bowel wrapped within a fibrotic capsule (Fig. 1). His symptoms continued for 10 days after presentation and he, therefore, underwent surgical treatment. During the laparotomy, a thick fibrous membrane wrapping around the small intestine and colon was revealed. Careful dissection and excision of the thick membrane were performed to release the small intestine without additional injury to the intestinal serosa (Fig. 2). After surgery, tamoxifen 20 mg was administered twice a day and FK was replaced with mTOR inhibitor (sirolimus, target trough level: 5–10 ng/mL). Prednisolone was restarted at 4 mg twice a day. He was able to start oral feeding 5 days after surgery and was discharged 2 weeks later without any complication. The tamoxifen was maintained for 3 months. He was symptom-free for 1 year with continued treatment with sirolimus and predenisone.
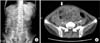
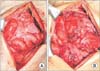
Case 2 was a 51-year-old man who suffered from end-stage liver disease caused by Budd-Chiari syndrome, diagnosed in 2005. He had 6 cases of esophageal variceal bleeding treated with rubber band ligation, but had no history of ascites drainage or SBP. He had a subtotal gastrectomy and Billoth-II anastomosis due to adenocarcinoma detected from the varix ligation specimen in 2012. He underwent living donor LT due to recurrent variceal bleeding with a left lobe graft from his daughter on August, 2014. During operation, fibrotic change of the peritoneum was not observed. His maintenance immunosuppressive agents were FK, MPA, and prednisone. His early posttransplant course was complicated by portal vein stenosis and a large amount of ascites that was treated with angiographic portal vein balloon dilatation and percutaneous drainage of the ascites. Klebsiella pneumoniae was cultured on the drained ascites and he was treated with ertapenem for 2 weeks. He was discharged 4 weeks after transplant without any complications. Three months after transplant, he was readmitted with nausea, vomiting, and abdominal pain without rebound tenderness. His upper gastrointestinal series revealed a distended stomach and jejunum with delayed passage. Abdominal CT revealed diffuse thickening of the visceral peritoneum of the small bowel loop with suspicious adhesions and decreased mural enhancement of the terminal ileum (Fig. 3). Tamoxifen 20 mg was administered twice a day and FK was replaced with mTOR inhibitor (sirolimus, target trough level: 5–10 ng/mL). Prednisolone was restarted at 4 mg twice a day. Two weeks after medical treatment, he complained of aggravated abdominal pain and a subjective fever. Follow-up CT revealed terminal ileum perforation, which was suspected due to decreased mural perfusion on the prior CT. He was taken back to the operating room and received a terminal ileum resection and anastomosis. Dissection of the fibrotic membrane and adhesiolysis were also performed (Fig. 4). He was able to start oral feeding 6 days after the operation and was discharged 4 weeks after the operation. Tamoxifen was maintained for 3 months, and he was continued on sirolimus and prednisone. He has maintained an oral diet without nausea or vomiting for 20 months after operation.
ESP is characterized by encasement of the small intestine by a thick fibrous membrane and a rare but possible complication in LT recipients. The etiology of ESP is unclear. The ‘two hit theory’ suggests that long-term inflammation of the peritoneum is needed in the pathogenesis of ESP [7]. SBP prior to transplantation, accumulation of ascites after transplantation, and postoperative hemorrhage could cause ESP after LT [5]. In these cases, a history of SBP was found only in case 1, but posttransplant intraperitoneal inflammation was found in both cases. Moreover, a previous study reported that the widespread use of CNI for maintenance immunosuppression is a factor contributing to the incidence of ESP after kidney transplantation [3]. The same situation applies for LT recipients. Therefore, clinicians who take care of LT recipients need to know about the possibility of ESP after LT when LT recipients complain of symptoms of small bowel obstruction such as nausea and vomiting.
Several medical treatments for ESP have been proposed. Corticosteroids have been successfully used [8]. Tamoxifen, a selective estrogen receptor modulator stimulates TGF-β1, which facilitates the removal of denatured collagen and has been used to treat ESP in previous studies [4]. However, the therapeutic dose and duration of treatment is still debatable. mTOR inhibitors are well-known for having antiproliferative effects and have recently been used as medical treatment for ESP after transplantation [9]. The synergistic effects of mTOR inhibitor and tamoxifen have also been reported [10]. However, the optimal dose and duration have not been determined. In this report, corticosteroids, tamoxifen, and mTOR inhibitors were used for medical treatments. To the best of our knowledge, this is the first case report of ESP after LT managed with these medications.
There is no optimal evidence-based treatment for ESP. However, surgical treatment is mandatory when there are symptoms of intestinal obstruction [5]. Stripping the thick fibrotic membrane is essential, but care should be taken not to tear the serosa of the intestine. Small bowel resection is necessary only when there is perforation or stenosis. In this report, surgical treatment was performed in both cases. In case 2, medical treatment alone was not effective for ESP. The initial abdominal CT revealed decreased mural perfusion of the terminal ileum, a situation in which operation should be considered. In addition, when ESP is suspected, abdominal CT should be checked to rule out the intestinal mural perfusion defect.
In conclusion, ESP is a rare, but possible complication after LT, especially after postoperative, intraperitoneal inflammation. Surgical treatment combined with medical treatment including corticosteroids, tamoxifen, and mTOR inhibitor is effective for managing ESP after LT. Further studies are needed to confirm the detailed treatment strategy.
Figures and Tables
Fig. 1
(A) Abdominal X-ray appearance was not like mechanical obstruction. (B) Contrast abdominal CT revealed large amount of ascites and small bowel wrapped with capsule.
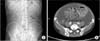
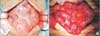
Fig. 2
(A) Surgical finding showed thick fibrous membrane wrapping the small intestine and colon. (B) Careful dissection and excision of the thick membrane to release the small intestine were performed without additional injury of the intestinal serosa.
References
1. Kawaguchi Y, Kawanishi H, Mujais S, Topley N, Oreopoulos DG. International Society for Peritoneal Dialysis Ad Hoc Committee on Ultrafiltration Management in Peritoneal Dialysis. Encapsulating peritoneal sclerosis: definition, etiology, diagnosis, and treatment. Perit Dial Int. 2000; 20:Suppl 4. S43–S55.
2. Korte MR, Sampimon DE, Betjes MG, Krediet RT. Encapsulating peritoneal sclerosis: the state of affairs. Nat Rev Nephrol. 2011; 7:528–538.
3. Korte MR, Yo M, Betjes MG, Fieren MW, van Saase JC, Boer WH, et al. Increasing incidence of severe encapsulating peritoneal sclerosis after kidney transplantation. Nephrol Dial Transplant. 2007; 22:2412–2414.
4. Khanna A, Plummer M, Bromberek C, Bresnahan B, Hariharan S. Expression of TGF-beta and fibrogenic genes in transplant recipients with tacrolimus and cyclosporine nephrotoxicity. Kidney Int. 2002; 62:2257–2263.
5. Mekeel K, Moss A, Reddy KS, Douglas D, Mulligan D. Sclerosing peritonitis and mortality after liver transplantation. Liver Transpl. 2009; 15:435–439.
6. Takeichi T, Narita Y, Lee KJ, Yamamoto H, Asonuma K, Inomata Y. Sclerosing encapsulating peritonitis after living donor liver transplantation: a case successfully treated with tamoxifen: report of a case. Surg Today. 2013; 43:1326–1329.
7. Honda K, Oda H. Pathology of encapsulating peritoneal sclerosis. Perit Dial Int. 2005; 25:Suppl 4. S19–S29.
8. Mori Y, Matsuo S, Sutoh H, Toriyama T, Kawahara H, Hotta N. A case of a dialysis patient with sclerosing peritonitis successfully treated with corticosteroid therapy alone. Am J Kidney Dis. 1997; 30:275–278.
9. Messina M, Ariaudo C, Mella A, Cantaluppi V, Segoloni GP, Biancone L. mTOR inhibitors for medical treatment of post-transplantation encapsulating peritoneal sclerosis: a favourable single center experience. J Nephrol. 2015; 28:245–249.
10. Huddam B, Azak A, Kocak G, Başaran M, Voyvoda N, Duranay M. Additive effectiveness of everolimus plus tamoxifen therapy in treatment of encapsulating peritoneal sclerosis. Ren Fail. 2012; 34:387–389.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download