Abstract
Purpose
Methods
Results
Figures and Tables
Fig. 1
All biliary cancer cell lines showed apurinic/apyrimidinic endodeoxyribonuclease1 (APEX1) expression by western blot. APEX1 protein expression was detected by western blot analysis with α-tubulin as a loading control.

Fig. 2
MTT assay for chemotherapeutic drugs (A: 5-FU, B: cisplatin, C: gemcitabine) in biliary cancer cells. Cell plated in 96-well plate were treated cisplatin, 5-FU, or gemcitabine and the MTT assay was carried out on day three of the treatments. Result are shown as means ± standard deviation (n = 3). 5-FU, 5-fluorouracil; MTT, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide.

Fig. 3
Western blot analysis of apurinic/apyrimidinic endodeoxyribonuclease1 (APEX1) expression in biliary cancer cell lines after APEX1 knockdown. SNU-245, SNU-308, SNU-478, SNU-1079, and SNU-1196 cells were transfected with control or APEX1-targeted siRNAs. Forty-eight hours after transfection, whole cell lysates were probed with antibodies specific for APEX1 and α-tubulin.

Fig. 4
(A) All biliary cancer cell line showed apurinic/apyrimidinic endodeoxyribonuclease1 (APEX1) and Jagged1 expression by Western blot. APEX1 and Jagged1 protein expression was detected by western blot analysis with α-tubulin as a loading control. (B) Western blot analysis of APEX1 and Jagged1 expression in biliary cancer cell lines after APEX1 knockdown. Western blot of all biliary cancer cells treated with APEX1 siRNA. Samples were collected 48 hours after APEX1-siRNA treatment, and Western blot was done with APEX1 antibody and reproved with α-tubulin antibody as a loading control.
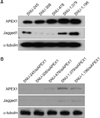
Fig. 5
Immunohistochemistry of advanced biliary cancer patients. Correlation between apurinic/apyrimidinic endodeoxyribonuclease1 (APEX1) and Jagged1 expression in biliary cancer patients. APEX1 and Jagged1 proteins in biliary cancer patients are shown by immunohistochemistry with anti-APEX1 and anti-Jagged1 antibodies. Brown staining indicates positive APEX1 or Jagged1 staining (P < 0.01, Pearson correlation test. Scale bars, 200 µm).

Fig. 6
Immunohistochemistry scoring of advanced biliary cancer patients. Apurinic/apyrimidinic endodeoxyribonuclease1 (APEX1) and Jagged1 expression levels assessed by immunohistochemistry scoring (see METHODS section).

Table 1
MTT assay for chemotherapeutic drugs (5-FU, gemcitabine, and cisplatin) in biliary cancer cells

Table 2
APEX1-speciifc siRNA-transfected biliary cancer cells were treated with chemotherapeutic drugs (5-FU, gemcitabine, and cisplatin, and MTT assay was performed)

Values are presented as drug concentration (%).
Drug concentrations which lead to a 50% inhibition of cell growth (IC50) were determined for each cell line using 3 chemotherapeutic drugs.
APEX1, apurinic/apyrimidinic endodeoxyribonuclease1; 5-FU, 5-fluorouracil; MTT, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide.
Table 3
The characteristic of ten biliary cancer patients

Apurinic/apyrimidinic endodeoxyribonuclease1 (APEX1) and Jagged1 expression levels assessed by immunohistochemistry scoring (see METHODS section). The 10 patients consisted of 2 groups according to the initial chemotherapy response (chemoresponsive patients; partial response [PR] vs. chemorefractory patients; progressive disease [PD]). FP; 5-FU plus cisplatin; GP, gemcitabine plus cisplatin.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download