Abstract
We present the case of a 31-year-old woman with an inflammatory and ulcerative malignant phyllodes tumor in her right breast. A right modified radical mastectomy and transverse rectus abdominis myocutaneous (TRAM) flap were performed. A month after the initial operation, several masses recurred at the superior margin and deep margin of the TRAM flap. Wide excision was performed, but masses recurred at the inferior margin and in both lung fields 2 weeks after the second operation. Six weeks after the second operation, the patient died due to progression of dyspnea and respiratory failure.
Phyllodes tumors are rare and account for 0.3 to 1% of all breast malignancies [1]. Malignant phyllodes tumors are classified according to the degree of stromal cellular atypia and represent 10% to 30% of all phyllodes tumors. There are some reports on malignant phyllodes tumors with an excellent prognosis, but few reports on aggressive phyllodes tumors that lead to death. We present a case of malignant phyllodes tumor with a fulminant course.
A 31-year-old woman from Uzbekistan presented with an inflammatory and ulcerative mass in her right breast that had been there since 2011. The mass had grown rapidly over the past 3 months and it was extremely painful with a huge ulcerative wound (Fig. 1A). Breast ultrasonography and a core needle biopsy were performed initially in an outside breast clinic and the biopsy result was a diagnosis of invasive carcinoma of no specific type. She was referred to Korea University Medical Center, Ansan in January 2016, 2 weeks after being diagnosed.
Additional imaging workup was performed to check the primary breast mass and metastatic lesion, including a MRI of the breast; a CT scan of the chest and abdomen; a bone scan; and a PET scan. Breast MRI revealed an irregular necrotic 120-mm mass in the right upper outer quadrant that had invaded the skin and pectoralis major muscle. Several lymph node (LN) metastases at the right axillary level I were suggested on the CT and PET scans, but there was no evidence of distant metastasis.
She underwent neoadjuvant chemotherapy with doxorubicin and cyclophosphamide for reduction of the mass and systemic therapy. After the first cycle of neoadjuvant chemotherapy, a core needle biopsy was performed again in our hospital, since our pathologist had suggested the possibility of a phyllodes tumor through review of the outside slides. The rebiopsy result was a diagnosis of phyllodes tumor, and a right modified radical mastectomy (MRM) and transverse rectus abdominis myocutaneous (TRAM) flap were performed (Fig. 1B). The right breast mass weighed 2,212 g at the time of excision.
The final pathology confirmed a malignant phyllodes tumor measuring 125 mm × 120 mm. The tumor showed brisk stromal overgrowth with lymph and vascular tumor emboli, and the mitotic count was 46 per 10 high power fields. All resection margins were negative and we obtained margins of more than 10 mm except for the deep resection margin which was less than 1 mm. Immunohistochemical staining for estrogen receptor (ER) and progesterone receptor (PR) were positive, but for human epidermal growth factor receptor 2 was negative. There was no metastasis in the 18 retrieved LNs.
A month after the initial operation, several mass-like protruding lesions appeared at the superior margin of the TRAM flap (Fig. 1C). Breast MRI showed recurrent tumors in the deep margin of the TRAM flap as well as the superior margin. The recurrent tumor in the deep margin measured 84 mm × 48 mm and had invaded the pectoralis minor and intercostal muscles (Fig. 2A, B). A wide excision was performed and a superior negative margin was obtained but a negative deep margin could not be achieved.
Two weeks after the second operation, masses appeared again at the inferior margin and a chest CT scan revealed new round opacities in both lung fields that were thought to be metastases (Fig. 2C). Despite palliative radiation therapy, bilateral pleural effusion increased gradually and the patient suffered from dyspnea. Six weeks after the second operation, the patient died due to progression of dyspnea and respiratory failure.
Phyllodes tumors are rare fibroepithelial breast tumors that usually present as a rapidly growing breast lump. The tumors are classified as benign, borderline, or malignant, but distinguishing among these histotypes is difficult. The classification is based on semiquantitative evaluation of mitotic activity, infiltrative as compared to circumscribed tumor margins, and the presence of stromal overgrowth [2]. The variable biologic behavior of phyllodes tumors has led to many debates about the treatment options.
A complete surgical excision is generally the treatment of choice for a phyllodes tumor [3]. Obtaining a negative histologic margin of at least 1 cm is suggested [4], but a recent retrospective study demonstrated that it was important to obtain a negative margin regardless of the length [5]. In our case, all histologic margins were negative for more than 1 cm except the deep margin at the initial operation. Nevertheless, the tumor recurred on the superior and inferior margin of the TRAM flap as well as the deep margin. This means that recurrence of aggressive malignant phyllodes tumors can be influenced by not only a negative margin, but also histologic grade, such as stromal overgrowth, high mitotic index, sarcomatous stroma, and an infiltrative margin.
Axillary LN metastases of phyllodes tumor are only rarely reported and axillary LN dissection is usually not required [3]. Although our patient was diagnosed with a phyllodes tumor, axillary LN dissection was performed because LN metastases were strongly suggested based on the chest CT and PET scan. No metastasis was found among the 18 retrieved LNs despite the aggressive activity of the disease. Thus, axillary LN dissection for phyllodes tumors should be carefully considered.
Radiation therapy decreases local recurrence of phyllodes tumor in the adjuvant setting [6]. However, it is difficult to directly assess the effectiveness of radiation therapy since previous studies enrolled patients with a negative resection margin at the initial operation. Furthermore, there has been no study on metastatic phyllodes treated with radiation therapy because of its rarity. In our case, the recurrent tumors after the second operation were not controlled by radiation therapy. The benefit of radiation therapy for aggressive malignant phyllodes tumors is hence questionable.
Moreover, the effectiveness of chemotherapy is controversial. A 10-year observational study of adjuvant chemotherapy for malignant phyllodes tumor demonstrated that there was no benefit for patients from treatment with doxorubicin and dacarbazine [7]. However, according to a study of soft tissue sarcoma, neoadjuvant chemotherapy including ifosfamide is feasible to treat sarcoma [8]. Our patient could not undergo chemotherapy because of her poor general condition caused by progressive dyspnea.
Hormone therapy is not effective for malignant phyllodes tumors even when they are positive for ER and PR [9] because hormone receptors in phyllodes tumor are located mainly in the epithelial component, not in the stromal component [10]. In our case, the patient did not undergo hormone therapy despite being positive for ER and PR.
In conclusion, aggressive malignant phyllodes tumor remains a therapeutic challenge despite extensive research, and further studies are required. Physicians should be aware that malignant phyllodes tumor can progress to a fulminant course.
Figures and Tables
Fig. 1
(A) An inflammatory and ulcerative mass in the right breast. (B) Post modified radical mastectomy image showing en bloc removal of the tumor including the invaded pectoralis muscle. (C) Mass-like protruding lesions that appeared at the superior margin of the transverse rectus abdominis myocutaneous flap a month after the initial operation.
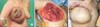
Fig. 2
(A, B) Breast MRI showing recurrent tumor (84 mm × 48 mm) at the deep margin of the transverse rectus abdominis myocutaneous flap invading the pectoralis minor and intercostal muscles, a month after the initial operation. (C) A chest CT scan for both lung fields showing new round opacities and suggesting metastases (arrows).

References
1. Geisler DP, Boyle MJ, Malnar KF, McGee JM, Nolen MC, Fortner SM, et al. Phyllodes tumors of the breast: a review of 32 cases. Am Surg. 2000; 66:360–366.
2. Kim S, Kim JY, Kim DH, Jung WH, Koo JS. Analysis of phyllodes tumor recurrence according to the histologic grade. Breast Cancer Res Treat. 2013; 141:353–363.
3. Macdonald OK, Lee CM, Tward JD, Chappel CD, Gaffney DK. Malignant phyllodes tumor of the female breast: association of primary therapy with cause-specific survival from the Surveillance, Epidemiology, and End Results (SEER) program. Cancer. 2006; 107:2127–2133.
4. Rowell MD, Perry RR, Hsiu JG, Barranco SC. Phyllodes tumors. Am J Surg. 1993; 165:376–379.
5. Jang JH, Choi MY, Lee SK, Kim S, Kim J, Lee J, et al. Clinicopathologic risk factors for the local recurrence of phyllodes tumors of the breast. Ann Surg Oncol. 2012; 19:2612–2617.
6. Barth RJ Jr, Wells WA, Mitchell SE, Cole BF. A prospective, multi-institutional study of adjuvant radiotherapy after resection of malignant phyllodes tumors. Ann Surg Oncol. 2009; 16:2288–2294.
7. Morales-Vasquez F, Gonzalez-Angulo AM, Broglio K, Lopez-Basave HN, Gallardo D, Hortobagyi GN, et al. Adjuvant chemotherapy with doxorubicin and dacarbazine has no effect in recurrence-free survival of malignant phyllodes tumors of the breast. Breast J. 2007; 13:551–556.
8. Gortzak E, Azzarelli A, Buesa J, Bramwell VH, van Coevorden F, van Geel AN, et al. A randomised phase II study on neo-adjuvant chemotherapy for ‘high-risk’ adult soft-tissue sarcoma. Eur J Cancer. 2001; 37:1096–1103.
9. Telli ML, Horst KC, Guardino AE, Dirbas FM, Carlson RW. Phyllodes tumors of the breast: natural history, diagnosis, and treatment. J Natl Compr Canc Netw. 2007; 5:324–330.
10. Tse GM, Lee CS, Kung FY, Scolyer RA, Law BK, Lau TS, et al. Hormonal receptors expression in epithelial cells of mammary phyllodes tumors correlates with pathologic grade of the tumor: a multicenter study of 143 cases. Am J Clin Pathol. 2002; 118:522–526.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download