Abstract
Focal nesidioblastosis is a rare cause of endogenous hyperinsulinemic hypoglycemia in adults. Because it is difficult to localize and detect with current imaging modalities, nesidioblastosis is challenging for biliary-pancreatic surgeons. 68Gallium-DOTA-D-Phe1-Tyr3-octreotide PET scanning and 111indium-pentetreotide diethylene triamine pentaacetic acid octreotide scanning may be superior to conventional imaging modalities in determining the localization of nesidioblastosis. We report the successful surgical treatment of a 54-year-old woman with focal hyperplasia of the islets of Langerhans, who experienced frequent hypoglycemic symptoms and underwent various diagnostic examinations with different results.
Endogenous hyperinsulinemic hypoglycemia (EHH) is a condition of hypoglycemia due to excessive endogenous insulin in the absence of injected exogenous insulin and drugs. The most frequent cause of EHH in neonates is genetic, characterized by the diffuse or focal hypertrophy and hyperplasia of β-cells [1]. This condition, called nesidioblastosis, was first described in 1938 to explain the islets (nesidioblasts) resulting from the diffuse proliferation of cells differentiating from duct epithelium [2]. In adults, insulinoma is the most common cause of EHH and the most common hormone-active endocrine tumor of the pancreas. Nesidioblastosis in adults is rare, with an estimated incidence of approximately 0.5%–7% of all adults with organic hyperinsulinism [3].
Nesidioblastosis is difficult to diagnose clinically based on preoperative imaging modalities and patient symptoms. Despite improvements in the resolution of imaging modalities and functional imaging techniques, some of these lesions show faint or negative findings preoperatively [3]. Preoperative localization of nesidioblastosis, however, is important for surgical resection procedures.
This report describes a patient with focal nesidioblastosis who showed different results on various preoperative imaging modalities and underwent surgical resection of the nesidioblastosis.
A 54-year-old woman with a 20-year history of hypoglycemic symptoms was referred to Seoul National University Hospital. Although she often fainted from hypoglycemia after light exercise or fasting, she had no symptoms before breakfast or at dawn (blood glucose level, 50–60 mg/dL) except for chronic fatigue and intermittent headache. Symptoms resolved spontaneously without eating. She had no relevant medical history or family history of diabetes. Physical examination was normal.
Blood tests at a previous hospital showed a blood glucose concentration after a 12-hour fast of 42 mg/dL (reference range, 70–110 mg/dL), an insulin concentration of 4.0 µIU/mL (reference range, 2–25 µIU/mL), a proinsulin concentration of 3.2 pmol/L (reference range, 6.7–26.5 pmol/L) and a C-peptide concentration of 1.85 ng/mL (reference range, 0.8–4.0 ng/mL). Her anti-insulin autoantibody concentration was 5.7% (reference range, 0%–7%) and her antiglutamic acid decarboxylase II antibody concentration was 0.46 units/mL (reference range, <0.9 units/mL). Her symptoms of hypoglycemia resolved after administration of dextrose.
Upon transfer to our institute, a 72-hour fasting test was attempted but had to be stopped after 6 hours because she experienced symptoms of hypoglycemia. Her blood glucose concentration was 35 mg/dL, her insulin concentration was 15.5 µIU/mL, her C-peptide concentration was 2.6 ng/mL, and her anti-insulin autoantibody concentration was 4.8%.
Pancreatobiliary-protocol CT scanning, MRI, and endoscopic ultrasonography showed no focal lesions in her pancreas (Fig. 1). Selective intra-arterial calcium stimulation with hepatic vein sampling (SAVS) showed different results in our hospital compared to those obtained in the previous hospital (Fig. 2). At the previous hospital, insulin concentration was higher in the splenic artery (SA) than in the superior mesenteric artery (SMA) and gastroduodenal artery (GDA). At our hospital, insulin concentration was higher in the SMA than in the SA and GDA. These results suggested that suspicious lesions would be located in areas supplied by the SMA or SA, that is, in the body or tail of the pancreas.
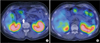
68Gallium-DOTA-D-Phe1-Tyr3-octreotide (DOTATOC) PET scanning showed increased DOTATOC uptake in the pancreas head area (Fig. 3), while 111indium-pentetreotide diethylene triamine pentaacetic acid octreotide (DTPAOC) scanning showed mildly increased octreotide uptake in the head of the pancreas (Fig. 4).
Although many attempts were made to localize the lesion, results were not in agreement with each other. No mass could be palpated during surgery by bimanual palpation. Intraoperative Ultrasound (US) failed to show a definite mass-like lesion in the pancreas, although subtle echoic changes were observed in the pancreas head. Based on the results of preoperative imaging, the surgeon decided to perform pylorus-preserving pancreatoduodenectomy.
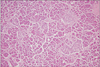
Pathologic examination of the resected specimen showed hyperplasia of the ductulo-insular complex in the focal area of the pancreas. Nuclear hyperchromasia and enlargement of β-cells were also found in that focal area, but there was no evidence of localized aggregation (Fig. 5).
Following the operation, the patient no longer experienced symptoms of hypoglycemia and her blood glucose level was increased to euglycemic levels.
Diffuse or focal nesidioblastosis in adults inducing EHH is a very rare disease. Although some cases of nesidioblastosis in adults have been reported, little is known about this disease. Preoperative diagnosis and localization remain difficult problems [3].
The clinical manifestations of nesidioblastosis are the same as other diseases that induce EHH, ranging from mild dizziness to life-threatening hypoglycemic attacks. The histopathologic features of nesidioblastosis include hypertrophic β-cells with pleomorphic nuclei, increased numbers of ductuloinsular complexes and neoformation of islets from ducts. Two forms of nesidioblastosis have been described: diffuse and focal. Although focal nesidioblastosis is frequently found in neonates, it is rarer than the diffuse form in adults [4]. In most adults, nesidioblastosis has unknown causes, which are not associated with genetic defects, as in neonates.
Although confirming a diagnosis of nesidioblastosis requires histopathologic examination, preoperative localization of a mass lesion is necessary for proper surgical resection procedures. In many patients, nesidioblastosis cannot be detected by current noninvasive imaging modalities, including US, CT, and MRI. Therefore, more specific modalities are required, including selective intra-arterial calcium stimulation with hepatic vein sampling (SAVS) and somatostatin receptor scintigraphy (SRS).
At present, SAVS is useful preoperatively for guiding surgical resection in patients with EHH. SAVS, which was developed in 1989, is based on the theory that calcium stimulates the release of insulin from hyperfunctioning β-cells of the pancreas [5]. Following selective arteriography, lesions in patients with EHH can be detected by injecting calcium gluconate into the major vessels of the pancreas including GDA, proper hepatic artery (PHA), SA, and SMA. A pathologic increase in serum insulin concentration can be detected among the involved regions, including the head, body and tail of the pancreas, by sampling blood from the hepatic venous effluent 0 (before injection), 30, 60 and 120 seconds after a bolus injection of calcium. A greater than 2- or 3-fold increase in serum insulin concentration indicates the presence of abnormal β-cells in that territory. If there are no anatomical variants, increased insulin in the GDA and SMA can indicate pancreatic head/neck lesions; whereas increased insulin in the SA and PHA can indicate pancreatic body/tail lesions and liver metastasis, respectively [6]. In diffuse nesidioblastosis, calcium injection may release insulin from the entire pancreas. The sensitivity and accuracy of SAVS in nesidioblastosis have not been determined yet, whereas in patients with insulinoma, the mean sensitivity of SAVS was found to be >90% (range, 62.5%–100%) and the accuracy to be 88%–94% [7]. False-negative results can include anatomical variants, vascular problems (e.g., celiac stenosis, splenorenal shunt) and technical or laboratory errors. False-positive results can include central tumor necrosis and abundant calcification [6]. Care should be taken in interpreting the results of SAVS. In our patient, repeat SAVS yielded different results, making complementary analysis necessary.
Functional imaging modalities based on the binding of radio-labeled analogues to somatostatin receptors (SSTR) have also been used to localize lesions that induce EHH. The use of SRS depends on their specific affinities to SSTR. SSTR subtypes 2 and 5 were reported to have high affinity, whereas subtype 3 was found to have moderate affinity [8]. Although SRS has been widely used in the detection of neuroendocrine tumor (NET) and insulinoma, its application in nesidioblastosis remains unclear. Recently, 68Ga-DOTATOC PET scanning was found to be superior to 111In-DTPAOC scanning, which was a standard technique of SRS, with better resolution (3–6 mm vs . 10–15 mm) and facilitating the quantification of isotope uptake [9]. 68Gallium has a shorter half-life (68 min) than 111indium (2.8 days) and a higher affinity in binding to SSTR2 [10]. Previous studies have reported more efficient results with better resolution using 68Ga-DOTATOC PET than 111In-DTPAOC scanning, especially in the detection of small NET lesions [89]. Our patient was evaluated by both 68Ga-DOTATOC PET scanning and 111In-DPTAOC scanning, with the former showing a more strongly enhancing focus. These functional imaging modalities provided critical information for performing surgery on our patient. Also, Care should be taken in interpreting results in patients with nesidioblastosis, as false positive results were observed in patients with noninsulinoma pancreatogenous hypoglycemia syndrome (NIPHS) [3].
The patient described here was ultimately diagnosed with focal nesidioblastosis, a condition very rare in adults. Several preoperative modalities were tested to improve the accuracy of localizing pathologic lesions in the pancreas (CT, MRI, SAVS, and 68Ga-DOTATOC PET and 111In-DPTAOC scanning). SAVS showed different results when repeated. In contrast, 68Ga-DOTATOC PET scanning and 111In-DPTAOC scanning showed the same results, which helped guide surgical resections. 68Ga-DOTATOC PET scanning was more accurate and with higher resolution than 111In-DPTAOC scanning. Although complementary results from various imaging modalities are important in the preoperative localization of nesidioblastosis, 68Ga-DOTATOC PET scanning may be useful as a primary examination in localizing pancreatic lesions, as it is able to sensitively detect lesions, as in our patient, when current diagnostic modalities fail to localize lesions or show unclear results. Further studies are needed to fully assess the diagnostic abilities of 68Ga-DOTATOC PET scanning compared with modalities currently used in clinical practice.
Figures and Tables
Fig. 1
Representative features of noninvasive radiologic findings of focal nesidioblastosis. Pancreatobiliary-protocol CT scanning (A), MRI (B), and endoscopic ultrasonography (C) showed no abnormal lesions in the pancreas of our patient.
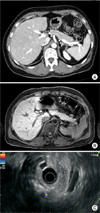
Fig. 2
Time-dependent changes of insulin concentraion in gastroduodenal artery, superior mesenteric artery and splenic artery, using selective intra-arterial calcium stimulation with hepatic vein sampling. (A) Our patient with focal nesidioblastosis had high insulin concentration in the superior mesenteric artery at our hospital. (B) At the previous hospital, insulin concentration was higher in the splenic artery than in the superior mesenteric artery and gastroduodenal artery.

Fig. 3
Representative features of 68gallium- DOTA-D-Phe1-Tyr3-octreotide (DOTATOC) PET scanning of focal nesidioblastosis. (A) Increased DOTATOC uptake in the pancreas head area was found clearly (white arrow). (B) No other abnormal lesion was found in the rest of the pancreas.
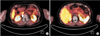
Fig. 4
Representative features of 111indium-pentetreotide diethylene triamine pentaacetic acid octreotide scanning of focal nesidioblastosis. (A) Increased octreotide uptake was found in the head of the pancreas, which was less clear than that of 68gallium- DOTA-D-Phe1-Tyr3-octreotide PET scanning (white arrow). (B) No other abnormal lesion was found in the rest of the pancreas.
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI14C2640).
References
1. Stanley CA, Lieu YK, Hsu BY, Burlina AB, Greenberg CR, Hopwood NJ, et al. Hyperinsulinism and hyperammonemia in infants with regulatory mutations of the glutamate dehydrogenase gene. N Engl J Med. 1998; 338:1352–1357.
2. Laidlaw GF. Nesidioblastoma, the islet tumor of the pancreas. Am J Pathol. 1938; 14:125–134.
3. Starke A, Saddig C, Kirch B, Tschahargane C, Goretzki P. Islet hyperplasia in adults: challenge to preoperatively diagnose noninsulinoma pancreatogenic hypoglycemia syndrome. World J Surg. 2006; 30:670–679.
4. McElroy MK, Lowy AM, Weidner N. Case report: focal nesidioblastosis ("nesidioblastoma") in an adult. Hum Pathol. 2010; 41:447–451.
5. Doppman JL, Shawker TH, Miller DL. Localization of islet cell tumors. Gastroenterol Clin North Am. 1989; 18:793–804.
6. Guettier JM, Kam A, Chang R, Skarulis MC, Cochran C, Alexander HR, et al. Localization of insulinomas to regions of the pancreas by intraarterial calcium stimulation: the NIH experience. J Clin Endocrinol Metab. 2009; 94:1074–1080.
7. Doppman JL, Miller DL, Chang R, Shawker TH, Gorden P, Norton JA. Insulinomas: localization with selective intraarterial injection of calcium. Radiology. 1991; 178:237–241.
8. Srirajaskanthan R, Kayani I, Quigley AM, Soh J, Caplin ME, Bomanji J. The role of 68Ga-DOTATATE PET in patients with neuroendocrine tumors and negative or equivocal findings on 111In-DTPA-octreotide scintigraphy. J Nucl Med. 2010; 51:875–882.
9. Buchmann I, Henze M, Engelbrecht S, Eisenhut M, Runz A, Schafer M, et al. Comparison of 68Ga-DOTATOC PET and 111In-DTPAOC (Octreoscan) SPECT in patients with neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2007; 34:1617–1626.
10. Reubi JC, Schar JC, Waser B, Wenger S, Heppeler A, Schmitt JS, et al. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med. 2000; 27:273–282.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download