Abstract
Purpose
Currently, development of pulmonary thromboembolism (PTE) after surgery is frequently being followed by legal action in Korea, as consequences may be fatal. In the current study, we assessed possible countermeasures that medical teams can take when faced with conflicting opinions on responsibility for PTE.
Methods
A retrospective analysis of claims handled by the Supreme Court and subordinate courts, from 1999 to 2015, was performed. We analyzed the type of procedure, associated complications, and critical legal points from the recorded judgments along with any liability limitations on surgeons.
Results
After reviewing cases between 1999 and 2015, a total of 18 cases were analyzed. There were no cases in which the surgeon was held accountable between 1999 and 2002. From 2003, there were instances of the surgeon being held accountable, with a peak of cases in 2013. Legal standards applied in judicial decision-making related to appropriate use of preventive measures, operation characteristics, doctor's reaction towards symptom occurrence, obligation of postoperative medical care, and duty of explanation.
Conclusion
The courts in Korea have changed their position from one of denying doctors' liability to one of enforcing responsibility for PTE. Surgeons are therefore being held responsible with greater frequency, depending on the details of the case. Lessons can be learnt from precedents that can be incorporated into medical education and training programs with the aim of reducing both major PTE complication rates and litigation costs.
Pulmonary embolism (PE) and deep venous thrombosis are clinical manifestations of the same disease, venous thromboembolic disease. Clinical diagnosis of PE is difficult in a significant percentage of patients and is frequently missed, explaining its high mortality rate [12]. In approximately 25% of patients, the first manifestation of PE is sudden-unexpected death [34]. Pulmonary thromboembolism (PTE) was major issue in the field of obstetrics because of its high frequency [5]. Pregnancy itself is a risk factor for PTE and the risk of PTE increases fivefold during pregnancy and 60-fold within 3 months after delivery [6]. Occurrences of PTE among 10,000 pregnancy and 10,000 postpartum cases are approximately 5–12 and 3–7, respectively [7]. All surgical departments, not just obstetrics, are now facing increasing difficulties associated with legal liability for PTE.
Currently, in Korea, PTE often leads to legal conflicts between doctors and patients, not only in obstetrics but in other surgery fields also. One reason for the increasing number of lawsuits is the finding that serious symptoms have been overlooked, such as the development of sudden chest pain and dyspnea, which can occasionally lead to death in otherwise asymptomatic patients. PTE is a major cause of morbidity and mortality in hospitalized patients. PTE was previously assumed to be uncontrollable, and surgeons often did not face legal responsibility because the symptoms are difficult to diagnose. Because of increasing evidence that patients with PTE can survive with appropriate preventive care, early diagnosis, and timely treatment, an increasing number of lawsuits related to PTE are successfully being brought on and won by patients in Korea. Surgeons are therefore being held responsible, depending on the details of the case. Doctors who avoid taking legal responsibility for related malpractice in such cases are no longer free from civil or criminal liability.
In surgery departments, PTE can be associated with a long duration of bed rest after a major surgery, such as major orthopedic surgery, breast reconstruction after mastectomy with flap surgery, or radical excision of a tumor. Despite thromboprophylactic measures, symptomatic PE remains a significant risk and a potentially lethal complication after major surgery [89]. Hence, surgeons in this field also should be prepared for legal problems arising from PTE [1011].
Three cases of postoperative PTE due to abdominal flap breast reconstruction were reported at Ewha Womans University Medical Center in the past 5 years. In those cases, total anesthesia time exceeded 10 hours because reconstruction was directly preceded by mastectomy. The average age of the patient population was 59 years. The presence of sufficient abdominal fat is a prerequisite for abdominal flap breast reconstruction. A long operating time, old age, and being overweight are known risk factors for PE [12]. Abdominal flap breast reconstruction carries with it several important risk factors for venous thromboembolism [1314]. However, the patient and/or his or her legal guardian may perceive the symptoms as a result of medical malpractice, even though symptoms appear uncontrollable from the doctor's point of view.
In the current study, we assessed possible countermeasures that medical teams can take when faced with conflicting opinions on responsibility for PTE. Through this research, we provide information that may help prevent conflicts and educate parties in medical disputes about PTE by evaluating judicial precedents in Korea on PTE development after surgery.
We assessed legal disputes arising from complications after surgery in Korea, including deep vein thrombosis, and PTE. Patients with simple deep vein thrombosis and without any subsequent effects did not take legal actions; therefore, studying such cases was not possible. Our current research was, thus, primarily concerned with cases of death or complications related to PTE after surgery in Korea. We searched law-related websites (Supreme Court Legal Information Service [glaw.scourt.go.kr] and LAWnB [www.lawnb.com] and Google Scholar) for completed lawsuits concerning thrombus formation related to surgery, except in the field of obstetrics between 1999 and 2015, and analyzed 18 cases for which case details were provided in full written judgment by the Supreme Court and subordinate courts. We assessed the following basic information: types of surgery, initial symptoms, liability of medical teams, legal decision-making factors, and reasons for which medical personnel were held responsible.
A total of 18 cases were analyzed and the following results were obtained. The causes of the claims were the major complications due to a delay in the diagnosis and or treatment of PTE.
Among the 18 cases of postoperative PTE, we categorized the disputes according to kinds of surgery procedures (Fig. 1). Among all cases, distribution of the categories was as follows: skin graft combined with burn injury 1 case (5.5%); abdominoplasty 1 case (5.5%); neurosurgery 2 cases (11.1%); radical excision of tumor 3 cases (16.6%); reconstructive flap surgery 5 cases (27.7%); major orthopedic surgery 6 cases (33.3%). The majority of procedures were orthopedic surgery due to hip, femur fracture, and lower extremity reconstruction with free flap due to extensive skin and soft tissue defect after trauma. Complications as a result of PTE were death in 9 cases (50%), ischemic cerebral palsy with vegetative state in 6 cases (33.3%), and other complications such as aesthetic dissatisfaction (because of deformities or scarring) in 3 cases (16.7%) (Fig. 2).
PTE is associated with a poor prognosis because of the difficulty of diagnosis, high mortality rate, and the fact that 90% of deaths resulting from PTE occur suddenly, within 1–2 hours [15]. It is the duty of the medical team to consider or predict the possibility of PTE and to perform all necessary evaluations to identify the cause of initial symptoms in cases where PTE appears.
We investigated the initial symptoms in the target cases and the countermeasures taken by the medical teams, and the results are shown in Fig. 3. The patients reported unstable vital sign (39%), general weakness (17%), pain (17%), chest discomfort (16%), abrupt dyspnea (11%) at the initial stage. When there were initial symptoms that doctors suspected as PTE, the doctors' management generally dictated legal liability. Thus, medical teams were not held liable for the occurrence of PTE if they recommended cardiothoracic treatment or hospitalization immediately after a patient reported symptoms. In contrast, surgeons who did not suspect PTE and did not take any measures or who recommended simple blood transfusions after misdiagnosing the condition as anemia were held responsible for malpractice.
After reviewing cases between 1999 and 2015, there was no case in which the surgeon was held accountable between 1999 and 2002. From 2003, there were instances of the surgeon being held accountable, with a peak occurrence of cases in 2013. Findings of nonaccountability remained low compared with findings of accountability from 2005 (Fig. 4), showing an upward trend in accountability.
In Korea, legal responsibility can be mitigated to some extent even when surgeons are found to be liable. Liability in the Korean court system is considered on a scale from 0% to 100%, with 100% equaling complete liability. As social norms have changed, the percentages of liability in cases have increased. Cases of 50%–100% liability were found to increase in frequency after 2005 (Fig. 5). Judgments put less legal responsibility on surgeons in relation to other medical lawsuits because asymptomatic cases of PTE may be difficult to diagnose, PTE may not always be preventable even when correct assessments are made and preventive treatment started, and death cannot always be prevented even when PTE is detected early and adequate treatment is initiated based on symptoms. Increasing awareness of the characteristics of preventable PTE cases is reflected in changing legal judgments.
The standards applied to legal decision-making determining a surgeon's liability in this study can be categorized into 7 groups (Fig. 6): (1) whether the surgeon performed the operation with due care to prevent an embolic event, examined in the context of a violation of the duty of care; (2) postoperative care for monitoring of vital signs; (3) inadequate emergency treatment; (4) inadequate laboratory and radiological diagnostic procedures to confirm PTE; (5) no referral to tertiary hospital for management; (6) no proper explanation regarding the occurrence of PTE before the operation; and (7) whether the surgeon had proper equipment or management to prevent PTE.
From our analysis of cases, mistakes by the surgeon and late diagnosis of PTE were the main causes for findings of liability in 2005 and 2006. In the past 5 years, however, violation of the duty of explanation and inadequate preventive measures were the main causes of liability.
The standards applied to legal decision-making determining a surgeon's nonliability can be categorized into 8 groups (Fig. 7): (1) inevitable characteristics of PTE; (2) appropriate diagnostic examination; (3) appropriate post-operative care; (4) appropriate symptomatic management; and (5) acknowledgement that the situation was undetectable because the patient did not present with any clinical symptoms. After 2005, new categories appeared; these are: (6) whether the patient had predisposing risk factors such as obesity and old age; (7) whether equipment/procedures for embolic event prevention were adequately provided, such as pneumatic compression or air mattresses; and (8) the encouragement of early ambulation. Whether adequate explanations were given regarding the possibility of complications is a major factor in liability, regardless of timing.
Development of PTE is partly beyond the control of the surgeon and the occurrence of PTE is not necessarily indicative of malpractice by the medical team. In the past, it was assumed that the occurrence of PTE was inevitable because of its characteristics. After reviewing cases from 1999, 2001, and 2002, we found that the unchangeable and uncontrollable properties of postoperative PTE were the main reasons for findings of nonliability for plastic surgeons.
PTE can be caused by deep vein thrombosis and it is usually caused by venous stasis, endothelial injury, or hypercoagulability [16]. Although genetic factors affect the development of PTE, the condition occurs most commonly during pregnancy, childbirth, surgery, and with tumors. Because diagnosis is delayed in many cases owing to frequent lack of discernable symptoms, PTE has high morbidity and mortality rates. According to the American College of Chest Physicians Clinical Practice Guidelines on Antithrombotic and Thrombolytic Therapy, even if a timely diagnosis and proper treatment are ensured, it is very difficult to prevent fatal complications and death [17].
In the past, no special preventive measures for PTE were implemented during surgery. Following the accumulation of evidence suggesting a reduced frequency of PTE after preventive measures, attempts to prevent PTE have increased [18]. PTE prophylaxis and treatment have received increasing attention in surgery [19]. For the treatment of venous thromboembolism (VTE), thromboprophylaxis has been recommended based on the four following factors: the high incidence of VTE in hospitalized patients; the difficulty of early diagnosis due to vague symptomatology; the cost-effectiveness of medical prophylaxis; and the high mortality of PE without early diagnosis and prompt management [20].
Skin-sparing mastectomy and breast reconstruction for breast cancer patients are being increasingly perceived as operations associated with a high risk for PTE. Considering that the underlying disease is cancer, these procedures involve many surgical hours, and necessitate bed rest for flap stabilization and pain relief. In a prospective study for postsurgical PTE, Lee et al. [21] reported that 20.4% of patients who underwent skin-sparing mastectomy and immediate reconstruction with a rectus abdominis myocutaneous flap developed PTE during the recovery period. Iorio et al. [11] also emphasized the need for preventive measures for PTE associated with elective surgeries such as abdominoplasty and liposuction.
There are no reports focusing on the frequency of litigation due to PTE associated with surgery. To our knowledge, major surgery is associated with various risk factors for PTE; therefore, every surgeon should be prepared for the possibility of postsurgical PTE and malpractice litigation due to PTE. For example, recently, there has also been increased concern about postsurgical PTE with plastic surgery [22]. Reflecting this trend, the American Society of Plastic Surgeons Venous Thromboembolism Task Force established the Caprini risk-assessment score using patient-risk stratification. According to risk assessment scoring, breast reconstruction and soft tissue reconstructive surgeries, which are frequently performed by plastic surgeons, pose a high risk for PTE because they require more than 6 hours of operating time and a prolonged period of bed rest to ensure pedicle stability of the free flap. Plastic surgeons should assess risk factors for PTE using a risk scoring system prior to operating and implement proper preventive measures. This does not indicate that every patient needs a thorough presurgical inspection and treatment with an anticoagulant, but rather suggests that doctors need to be careful in observing the patient's symptoms and pay close attention to nonspecific symptoms. Explaining the risks of PTE occurrence and taking preventative measures is not a choice but a duty of every surgeon.
In the legal context, the courts in Korea have changed their position from one of denying doctors' liability to one of enforcing responsibility for PTE. PTE was previously assumed to be uncontrollable because of the difficulty of diagnosis and of preventing the development and progression of symptoms. However, recently in Korea, accumulating evidence that patients with PTE can survive with appropriate preventive care, early diagnosis, and timely treatment, has led to an increasing number of lawsuits related to PTE being won by patients. Additionally, the courts have stated that medical teams need to be alert and prepared to implement proper measures to prevent or manage potential thrombus. The courts are now of the view that a doctor's duty of care includes foreseeable postsurgical risks, with that duty extending from immediately after the operation to the postsurgical recovery process. This change in thinking is related to increasingly high standards of care. Further, courts have begun to find legal liability on the part of medical teams as research on risk factors for PTE increases. Such research has provided clinical guidelines for various preventive measures for PTE, and suggests the possibility of a complete cure through early diagnosis and timely treatment.
In the current study, we assessed the legal decisions related to postsurgical PTE following surgery and confirmed the types of cases in which doctors were held liable. Through these cases, the courts have determined that medical teams must preoperatively assess risk factors for PTE, take adequate preventive measures, and follow proper treatment procedures when patients report symptoms such as acute respiratory arrest or chest pain. And when patients are understood to possess a high risk for PTE, the surgeons must give more thorough explanations regarding preventive measures such as early ambulation or pneumatic compression [2324].
We did find cases in which doctors were not held liable when patients did not have risk factors for susceptibility to PTE. In such cases, presurgical prediction was impossible. So, even if a patient had complications, when doctors managed postoperative PTE properly they were unaccountable for PTE.
Risk factors are not the only measure of liability. It is critical to remember that surgeons cannot avoid legal liability if they only explain the possibility of PTE without discussing postsurgical preventive measures such as early ambulation.
Prevention of PTE is understood to be more cost-effective than treatment, and various academic societies in the United States and Europe have issued recommendations focusing on prevention since the 1980s [2526]. However, each guideline has a different opinion and differs in topic, such as patient categorization and anticoagulation use [27]. A medical team may be confused about which guideline to follow, whether to use anticoagulation therapy according to the specific guideline regardless of hemorrhagic risk, and whether the team could avoid legal responsibility by following the guideline. Unfortunately, the cases examined in this study did not specify which guidelines should be followed.
Given that doctors bear the duty of care during medical practice, which is based on the requisite level of care in clinical medicine at the time, and that the level of care is a relative concept and is taken normatively by the court, the Department of Justice will require a reasonable basis if they are to select a guideline in the future.
In the examined cases, judges focused on whether the patient reported symptoms and the manner in which the doctors reacted to them. Doctors were held liable in all cases in which patients had reported dizziness, chest tightness, and chest discomfort, but doctors failed to suspect or assess PTE. The Department of Justice has stated that in cases of skin graft due to extensive burn injury, doctors should assess PTE and must suggest early ambulation to the patient and engage in a thorough inspection if the patient reports any symptoms. In contrast, courts did not find the medical team liable in cases where the patient refused additional treatment.
Patients can agree or disagree with the doctor's medical advice, but without proper medical knowledge, patients cannot make practical decisions. Doctors therefore bear the duty of explaining important factors so that a reasonable patient can make appropriate medical decisions. Factors to be explained include the patient's possible symptoms, the necessity and process of the surgical procedure, and risks associated with not following medical advice. Such explanations are aimed at protecting a patient's right to make their own decision by supplementing the patient's inexpert medical knowledge. Doctors do not have to explain factors that are common knowledge, and they cannot be held liable for not explaining factors that appear to be common sense when the patient refuses treatment while knowing the risks related to refusal.
The current legal precedent states that even if complications are a result of the doctor's malpractice, it is unreasonable for the hospital to be found 100% liable except in extreme cases; these include exceptionally critical malpractice and operations being performed by non-medical professionals. The reasons why doctors should not bear full liability are: (1) if doctors frequently bear full liability for medical practice they will become apprehensive and passive in practice; (2) in medical practices of a lifesaving nature, malpractice may not be attributable to the doctor; and (3) there are uncontrollable factors related to patients' physical characteristics and the limits of modern clinical medicine [28]. In some cases, courts have mitigated medical teams' liability because there are many cases of asymptomatic PTE; further, even though symptoms may appear, the rapid clinical process of PTE may make diagnosis and successful treatment extremely difficult.
As a result of our study, we found that the court's legal perception of the term “uncontrollable situation” about PTE has changed. According to the changed paradigm, the diagnosis and treatment of PTE is within the scope of medical practitioners' duty of care, despite the fact that the occurrence of PTE may be beyond control in many cases. Explaining the risks of PTE to patients as well as prevention measures, early diagnosis, and early treatment of PTE are critical in surgery and need to be considered by medical teams. Lessons can be learnt from precedents that can be incorporated into medical education and training programs with the aim of reducing both major PTE complication rates and litigation costs.
Figures and Tables
Fig. 2
Distribution of complications after diagnosis of pulmonary thromboembolism that mainly concerned medical lawsuits.
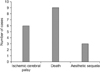
Fig. 3
Initial clinical manifestations before diagnosis of pulmonary thromboembolism. Distribution of surgery procedures in malpractice claims.
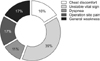
Fig. 4
Yearly distribution of courts' judgments in pulmonary thromboembolism-related plastic surgery malpractice claims.

References
1. Ermenc B. Minimizing mistakes in clinical diagnosis. J Forensic Sci. 1999; 44:810–813.
2. Goldhaber SZ. Pulmonary embolism. Lancet. 2004; 363:1295–1305.
3. Heit JA, Silverstein MD, Mohr DN, Petterson TM, Lohse CM, O'Fallon WM, et al. The epidemiology of venous thromboembolism in the community. Thromb Haemost. 2001; 86:452–463.
4. Heit JA. The epidemiology of venous thromboembolism in the community: implications for prevention and management. J Thromb Thrombolysis. 2006; 21:23–29.
5. Danilenko-Dixon DR, Heit JA, Silverstein MD, Yawn BP, Petterson TM, Lohse CM, et al. Risk factors for deep vein thrombosis and pulmonary embolism during pregnancy or post partum: a population-based, case-control study. Am J Obstet Gynecol. 2001; 184:104–110.
6. Iverson RE, Gomez JL. Deep venous thrombosis: prevention and management. Clin Plast Surg. 2013; 40:389–398.
7. New H, Byers CG. Pulmonary thromboembolism. Compend Contin Educ Vet. 2011; 33:E1.
8. Hofer SO, Damen TH, Mureau MA, Rakhorst HA, Roche NA. A critical review of perioperative complications in 175 free deep inferior epigastric perforator flap breast reconstructions. Ann Plast Surg. 2007; 59:137–142.
9. Spear SL, Ducic I, Cuoco F, Taylor N. Effect of obesity on flap and donor-site complications in pedicled TRAM flap breast reconstruction. Plast Reconstr Surg. 2007; 119:788–795.
10. Colwell AS, Reish RG, Kuter DJ, Damjanovic B, Austen WG Jr, Fogerty AE. Abdominal contouring procedures increase activity of the coagulation cascade. Ann Plast Surg. 2012; 69:129–133.
11. Iorio ML, Venturi ML, Davison SP. Practical guidelines for venous thromboembolism chemoprophylaxis in elective plastic surgery. Plast Reconstr Surg. 2015; 135:413–423.
12. Geerts WH, Pineo GF, Heit JA, Bergqvist D, Lassen MR, Colwell CW, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004; 126:3 Suppl. 338S–400S.
13. Lemaine V, McCarthy C, Kaplan K, Mehrara B, Pusic AL, Cordeiro PG, et al. Venous thromboembolism following microsurgical breast reconstruction: an objective analysis in 225 consecutive patients using low-molecular-weight heparin prophylaxis. Plast Reconstr Surg. 2011; 127:1399–1406.
14. Lee SH, Lee TJ, Eom JS, Son BH, Ahn SH, Lee SD. Incidence and risk factors of pulmonary thromboembolism in pedicled TRAM breast reconstruction. J Korean Soc Plast Reconstr Surg. 2006; 33:193–197.
15. Munteanu I. Current treatment of venous thrombembolism. Pneumologia. 2013; 62:37–42.
16. Stein PD, Henry JW. Prevalence of acute pulmonary embolism among patients in a general hospital and at autopsy. Chest. 1995; 108:978–981.
17. Janata K, Holzer M, Domanovits H, Müllner M, Bankier A, Kurtaran A, et al. Mortality of patients with pulmonary embolism. Wien Klin Wochenschr. 2002; 114:766–772.
18. Bak SM, Lee SH, Lee SH, Sin C, Cho JY, Shim JJ, et al. Clinical study fo pulmonary thromboembolism. Tuberc Respir Dis. 2001; 50:106–116.
19. Bang SM, Jang MJ, Kim KH, Yhim HY, Kim YK, Nam SH, et al. Prevention of venous thromboembolism, 2nd edition: Korean Society of Thrombosis and Hemostasis Evidence-based Clinical Practice Guidelines. J Korean Med Sci. 2014; 29:164–171.
20. Wes AM, Wink JD, Kovach SJ, Fischer JP. Venous thromboembolism in body contouring: an analysis of 17,774 patients from the National Surgical Quality Improvement databases. Plast Reconstr Surg. 2015; 135:972e–980e.
21. Lee JS, Son BH, Choi HS, Sung JM, Hong SJ, Kim JK, et al. Pulmonary thromboembolism following mastectomy with Immediate TRAM in the patients with breast cancer: a prospective study. J Breast Cancer. 2006; 9:354–360.
22. Partsch H. Therapy of deep vein thrombosis with low molecular weight heparin, leg compression and immediate ambulation. Vasa. 2001; 30:195–204.
23. Sevitt S, Gallagher N. Venous thrombosis and pulmonary embolism. A clinico-pathological study in injured and burned patients. Br J Surg. 1961; 48:475–489.
24. Clagett GP, Reisch JS. Prevention of venous thromboembolism in general surgical patients. Results of meta-analysis. Ann Surg. 1988; 208:227–240.
25. Salzman EW, Davies GC. Prophylaxis of venous thromboembolism: analysis of cost effectiveness. Ann Surg. 1980; 191:207–218.
26. Murphy RX Jr, Alderman A, Gutowski K, Kerrigan C, Rosolowski K, Schechter L, et al. Evidence-based practices for thromboembolism prevention: summary of the ASPS Venous Thromboembolism Task Force Report. Plast Reconstr Surg. 2012; 130:168e–175e.
27. Bak KH. A study on patient's obligation in medical cooperation and doctor's medical malpractice. Korean Soc Law Med. 2012; 13:91–123.
28. Stein PD, Matta F. Pulmonary embolism and deep venous thrombosis following bariatric surgery. Obes Surg. 2013; 23:663–668.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download