Abstract
Purpose
Livin is associated with drug response in several cancers. The aim of this study was to investigate the effect of silencing the livin gene expression on anticancer drug response in colorectal cancer.
Methods
siRNA was transfected at different concentrations (0, 10, and 30nM) into HCT116 cells, then cells were treated with either 5-fluorouracil (FU)/leucovorin (LV) or oxaliplatin (L-OHP)/5-FU/LV. Cellular viability and apoptosis were evaluated following silencing of livin gene expression combined with treatment with anticancer drugs.
Results
Livin gene expression was effectively suppressed by 30nM siRNA compared with control and 10nM siRNA. The 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assay showed that proliferation was effectively inhibited in cells treated with a combination of both siRNA and an anticancer drug, compared to cells treated with siRNA-Livin or anticancer drug alone. In particular, the combination of 30nM siRNA and L-OHP/5-FU/LV resulted in a 93.8% and 91.4% decrease, compared to untreated control or L-OHP/5-FU/LV alone, respectively. Cellular proliferation was most effectively suppressed by a combination of 30nM of siRNA and L-OHP/5-FU/LV compared to other combinations.
Conclusion
siRNA-mediated down-regulation of livin gene expression could significantly suppress colon cancer growth and enhance the cytotoxic effects of anticancer drugs such as 5-FU and L-OHP. The results of this study suggest that silencing livin gene expression in combination with treatment with anticancer drugs might be a novel cancer therapy for colorectal cancer.
Colorectal cancer is one of the most common malignant tumors worldwide and the incidence rate has steadily increased. It is the third leading cause of death in cancer patients [1]. The complete cure rate is 70% to 80% at diagnosis; however, metastasis develops in 50% of patients [1]. Therefore, systemic chemotherapy, in addition to surgical treatment, has been widely applied to improve the survival rate [2]. Currently, the commonly used regimens combine drugs, such as oxaliplatin (L-OHP) or irinotecan-based on 5-fluorouracil (FU). However, these treatments have not led to a satisfactory survival rate in metastatic colorectal cancer patients [1]. Therefore, new therapies have been developed to improve the treatment outcome for colorectal cancer patients. Targeted therapy has attracted attention as a potential new treatment option that targets apoptotic genes, oncogenes, and cell cycle regulatory genes.
The inhibition of apoptosis is one of the most important mechanisms in carcinogenesis. Apoptosis-related genes and proteins take a significant role in the growth and proliferation of cancer cells [3]. Inhibitor of apoptosis proteins (IAPs) is a typical anti-apoptotic protein that suppresses apoptosis by binding caspases and inhibiting proteolytic processes [4]. The most well-known IAPs are C-IAP1, C-IAP2, NAIP, Survivin, X-linked IAP (XIAP), Bruce, ILP-2, and Livin. Livin and Survivin are the most representative members. Of special interest is Livin, which is a member of the IAP family. Livin is abundantly expressed in cancer cells, but is rarely detected in normal cells. It has a high affinity for cancer cells, so it is expected to be a potential target of chemotherapy [5]. In this study, we aimed to investigate whether silencing the livin gene affects the antitumor effect of anticancer drugs in colorectal cancer.
The HCT116 colon cancer cell line was purchased from the Korean cell line bank. The cells were grown in RPMI1640 medium (Life Technologies Inc., Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Life Technologies Inc.), 100 U/mL of penicillin, and 100 g/mL of streptomycin at 37℃ in a humidified incubator under an atmosphere of 5% CO2. The HCT116 cells were seeded at a density of 1×105 cells per well in 6-well plates and transfected with different concentrations (10nM and 30nM) of siRNA (Dharmacon Inc., Lafayette, CO, USA) against the livin gene using Lipofectamine 2000 (Life Technologies Inc.) according to the manufacturer's instructions [6]. Reverse transcription-polymerase chain reaction (RT-PCR) was performed as in the previous study to verify target knockdown [6]. The primers used to amplify the livin gene were as follows: forward 5′-CTGGTCAGAGCCAGTGTTCC-3′, and reverse 5′-TCATAGAAGGAGGCCAGACG-3′, for internal control gene β–actin, forward 5′-GACCTGACTGACTACCTCATGAA-3′, reverse 5′-CTTCATGATGGAGTTGAAGGTAG-3′.
L-OHP, 5-FU, and leucovorin (LV) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Stock solutions of these drugs were prepared in sterile distilled water with filtration. We chose concentrations of these drugs that caused 80%–90% growth reduction when used alone for HCT116 cell line: 2.5 µM of L-OHP, 25 µM of 5-FU, and 25 µM of LV. Cells were exposed to FL regimen (5-FU/LV) or folfox regimen (L-OHP/5-FU/LV) at 18 hours after transfection.
Cell viability was examined by routine 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. The HCT116 cells were seeded at a density of 2×104 cells per well in 96-well plates with RPMI1640 in a final volume of 100 µL. On the following day, cells were treated with 10 or 30 nM of siRNA and incubated for 6 hours and then treated with L-OHP, 5-FU, and LV, and then incubated for 18 hours. For MTT assay, 5-mg/mL MTT solution was added into each well. Following incubation at 37℃ for 4 hours, the reaction was stopped by the addition of dimethyl sulfoxide. After the crystals dissolved, the absorbency of the samples was determined at 550 nm.
Additionally, we analyzed the apoptotic activity of caspase 3 and caspase 7. The HCT116 cells were seeded at a density of 1×105 cells per well in 6-well plates. The cells were then treated with 30nM of siRNA against livin, L-OHP/5-FU/LV, or siRNA with L-OHP/5-FU/LV as previously described. The degree of apoptosis was determined based on the expression of caspase 3 and caspase 7 measured using RT-PCR. The primers were as follows: caspase 3, forward 5′-GACTCTAGACGGCATCCAGC-3′ and reverse 5′-TGACAGCCAGTGAGACTTGG-3′, caspase 7, forward 5′-AGTGACAGGTATGGGCGTTC-3′ and reverse 5′-CGG CATTTGTATGGTCCTCT-3′.
Each experiment was repeated two or more times. Bands from RT-PCR were quantified with UN-SCAN-IT gel version 6.1 software (Silk Scientific Inc., Orem, UT, USA). mRNA levels were calculated using β-actin levels as the reference. Statistical comparisons between different treatments were analyzed by Mann-Whitney test and analysis of variance. All statistical analyses were performed using SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was considered when P-values were less than 0.05.
We verified that livin gene expression was suppressed by siRNA in our previous study [5]. In this study, siRNA was transfected at different concentrations (10 and 30nM) into HCT116 cells, and then livin gene expression was detected by RT-PCR 18 hours after transfection. Transfection of siRNA against livin was successful. Control HCT116 cells expressed livin mRNA and this expression decreased after siRNA transfection. Livin expression was effectively suppressed by 30nM siRNA compared with control and 10nM of siRNA (Fig. 1).
To identify whether silencing livin affected the antitumor effect of anticancer drugs, cells were transfected with siRNA against livin and treated with anticancer drugs. siRNA was transfected at different concentrations (0, 10, and 30nM) into HCT116 cells, and then cells were treated with either 5-FU/LV or L-OHP/5-FU/LV after 18 hours. To quantify the cellular viability, MTT assay was performed.
The MTT assay showed that the proliferation of cells treated with a combination of siRNA and anticancer drug was effectively inhibited, compared with cells treated with siRNA-Livin or an anticancer drug alone. Although the proliferation of cells treated with 5-FU/LV in combination with 10nM of siRNA was similar to cells treated with 5-FU/LV alone, the proliferation of cells treated with the 30nM siRNA combination was significantly inhibited. When cells were treated with L-OHP/5-FU/LV with 10nM of siRNA, cellular proliferation was inhibited by 20.9% compared to treating with L-OHP/5-FU/LV alone. The combination of 30nM of siRNA and L-OHP/5-FU/LV resulted in a 93.8% and 91.4% decrease compared to untreated control or L-OHP/5-FU/LV alone, respectively. As shown in Fig. 2, cellular proliferation was most effectively suppressed by a combination of 30nM of siRNA and L-OHP/5-FU/LV.
We investigated whether livin silencing with chemotherapy induces apoptosis, resulting in suppression of cellular proliferation. The expression of caspase 3 and caspase 7, common markers for apoptosis, was evaluated by RT-PCR. The expression of caspase 3 and 7 decreased more with the combination of 30nM siRNA and L-OHP/5-FU/LV compared with either siRNA or L-OHP/5-FU/LV alone (Fig. 3). This result suggests that livin silencing enhances the cytotoxic effect of anticancer drugs by inducing apoptosis.
In this study, we investigated the effect of a combination treatment of siRNA-Livin and anticancer drugs in colon cancer. Compared with single-agent treatments, the combination of the siRNA-Livin and anticancer drugs displayed significantly greater inhibition of growth in HCT116 cells. Above all, the proliferation of cells was remarkably inhibited by up to 93.8% compared to the untreated control when cells were treated with L-OHP/5-FU/LV with 30nM of siRNA. Our results showed that treatment with L-OHP/5-FU/LV and siRNA-Livin produced a synergistic effect in HCT116 cells.
Many studies have revealed that silencing the livin gene inhibits cell proliferation and induces apoptosis in lung cancer, gastric cancer, and liver cancer [78910]. Especially, some studies have investigated the role of Livin on drug response in several types of cancer. A study observed that knockdown of livin gene expression increased apoptosis in colon cancer cells that were resistant to several chemoagents [11]. In other studies, silencing of livin reduced drug resistance to chemotherapy in glioblastoma, melanoma, and lung cancer [121314]. In addition, several studies have reported a synergetic anticancer effect when silencing of the livin gene is combined with chemotherapy [1516171819].
Livin, a member of the IAP family, is composed of 280 amino acids and has a single baculovirus IAP repeat domain and a COOH-terminal RING domain [20]. Livin inhibits apoptosis by binding to caspase 3, caspase 7, and caspase 9, and protects cells from various proapoptotic stimuli [2021]. Livin has been the focus of cancer therapy because it is easy to handle due to its small size, and it is rarely detected in normal tissues [20]. siRNA transfection targeting specific genes is relatively easy and thus has been used widely as a therapeutic strategy for numerous diseases including cancer [22]. In our previous study, we reported the use of siRNA targeting of livin as a treatment for colon cancer [6]. We used siRNA against livin at different concentrations of 25, 50, 100, 150, and 200nM in HCT116 colon cancer cells, and then detected expression of livin mRNA at 18, 24, and 30 hours after transfection. We found that the expression of livin was effectively suppressed by 25nM of siRNA at 18 hours after transfection. In this study, we transfected siRNA-Livin at 10 and 30nM, and then detected livin mRNA levels at 18 hours after transfection.
Chemotherapy has contributed to the improvement in the survival rate of cancer patients, but toxicity and resistance are major obstacles to successful treatment. Exposure of cancer cells to a single cytotoxic agent can cause resistance to unrelated agents. Therefore, the combination of chemotherapy and other modalities is often attempted to enhance the efficacy of chemotherapy [23]. It is possible that combination therapy with siRNA-Livin may be useful in patients who have resistance to 5-FU- or L-OHP-based chemotherapy. In addition to the antitumor effect, the systemic toxicity of treatment should be considered. In our previous study using a mouse xenograft model [5], we observed no significant toxic effects, so it may be that siRNA targeting of livin can be used in colon cancer therapy without systemic toxic effects in vivo. Thus, a regimen with combined use of siRNA-Livin and anticancer drugs may emerge as a potent and safe strategy for the treatment of colorectal cancer.
There were some limitations in our study. We used one cell line and did not confirm in vitro results with in vivo experiments. We did not evaluate the toxic effect of combined treatments with siRNA-Livin and chemoagents, although we identified that there was no significant toxicity of siRNA-Livin in vivo in our previous study. In addition, we did not investigate the mechanism of the synergetic antiapoptotic effect of siRNA-Livin and chemoagents. However, we showed that the expression of caspase 3 and 7 decreased following siRNA treatment, which indicates that siRNA transfection against Livin induces caspase dependent apoptosis in colon cancer cells.
In conclusion, siRNA-mediated down-regulation of livin gene expression could significantly suppress colon cancer growth and enhance the cytotoxic effects of anticancer drugs such as 5-FU and L-OHP. The results of this study suggest that silencing the livin gene might be a novel cancer therapy when used in combination with anticancer drugs for treating colorectal cancer.
Figures and Tables
Fig. 1
Silencing of livin gene expression after siRNA transfection. Livin gene expression was effectively suppressed after transfection of 30nM siRNA in HCT116 cells. Livin mRNA detected by reverse transcription-polymerase chain reaction. Columns = means ± standard deviation (n = 3). *P < 0.05. **P < 0.01. ***P < 0.001.
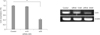
Fig. 2
Antiproliferative effect of silencing livin gene in combination with anticancer drug treatment. Proliferation of cells treated with combination of siRNA-Livin and anticancer drug was effectively inhibited. This effect was most prominent with combination of 30nM siRNA and oxaliplatin/5-fluorouracil/leucovorin. Cell viability was evaluated by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assay. Columns = means ± standard deviation (n = 3). *P < 0.05. **P < 0.01. ***P < 0.001.
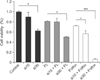
Fig. 3
Caspase activity after siRNA transfection and treatment with anticancer drugs. Expression of caspase 3 and 7 showed greater decrease in cells treated with combination of 30nM siRNA and oxaliplatin/5-fluorouracil/leucovorin (L-OHP/5-FU/LV) compared with either siRNA or L-OHP/5-FU/LV alone. Expression of caspase 3 and caspase 7 was evaluated by reverse transcription-polymerase chain reaction.

References
1. de Castro-Carpeno J, Belda-Iniesta C, Casado Saenz E, Hernandez Agudo E, Feliu Batlle J, Gonzalez Baron M. EGFR and colon cancer: a clinical view. Clin Transl Oncol. 2008; 10:6–13.
2. Oh SY, Kim DY, Suh KW. Oncologic outcomes following metastasectomy in colorectal cancer patients developing distant metastases after initial treatment. Ann Surg Treat Res. 2015; 88:253–259.
3. Bremer E, van Dam G, Kroesen BJ, de Leij L, Helfrich W. Targeted induction of apoptosis for cancer therapy: current progress and prospects. Trends Mol Med. 2006; 12:382–393.
4. Schimmer AD. Inhibitor of apoptosis proteins: translating basic knowledge into clinical practice. Cancer Res. 2004; 64:7183–7190.
5. Chang H, Schimmer AD. Livin/melanoma inhibitor of apoptosis protein as a potential therapeutic target for the treatment of malignancy. Mol Cancer Ther. 2007; 6:24–30.
6. Oh BY, Lee RA, Kim KH. siRNA targeting Livin decreases tumor in a xenograft model for colon cancer. World J Gastroenterol. 2011; 17:2563–2571.
7. Zhao X, Yuan Y, Zhang Z, Feng X, Zhang J, Yuan X, et al. Effects of shRNA-silenced livin and survivin on lung cancer cell proliferation and apoptosis. J BUON. 2014; 19:757–762.
8. Wang SL, Deng WT, Wen GF, Li CW, Zeng YJ. Construction of a Hep-2 cell line stably transfected with Livin shRNA. Bratisl Lek Listy. 2016; 117:272–275.
9. Liu P, Wang TS, You SH, Ge HM. Expression of livin in gastric cancer and effect of silencing of the livin gene on apoptosis in gastric cancer cells. Zhonghua Zhong Liu Za Zhi. 2007; 29:570–574.
10. Wang TS, Ding QQ, Guo RH, Shen H, Sun J, Lu KH, et al. Expression of livin in gastric cancer and induction of apoptosis in SGC-7901 cells by shRNA-mediated silencing of livin gene. Biomed Pharmacother. 2010; 64:333–338.
11. Wang X, Xu J, Ju S, Ni H, Zhu J, Wang H. Livin gene plays a role in drug resistance of colon cancer cells. Clin Biochem. 2010; 43:655–660.
12. Liu Y, Guo Q, Zhang H, Li GH, Feng S, Yu XZ, et al. Effect of siRNA-Livin on drug resistance to chemotherapy in glioma U251 cells and CD133+ stem cells. Exp Ther Med. 2015; 10:1317–1323.
13. Lazar I, Yaacov B, Shiloach T, Eliahoo E, Kadouri L, Lotem M, et al. The oncolytic activity of Newcastle disease virus NDV-HUJ on chemoresistant primary melanoma cells is dependent on the proapoptotic activity of the inhibitor of apoptosis protein Livin. J Virol. 2010; 84:639–646.
14. Sun JG, Liao RX, Zhang SX, Duan YZ, Zhuo WL, Wang XX, et al. Role of inhibitor of apoptosis protein Livin in radiation resistance in nonsmall cell lung cancer. Cancer Biother Radiopharm. 2011; 26:585–592.
15. Yu L, Wang Z. Effects of Livin gene RNA interference on apoptosis of cervical cancer HeLa cells and enhanced sensitivity to cisplatin. J Huazhong Univ Sci Technolog Med Sci. 2009; 29:625–630.
16. Wang R, Lin F, Wang X, Gao P, Dong K, Zou AM, et al. Silencing Livin gene expression to inhibit proliferation and enhance chemosensitivity in tumor cells. Cancer Gene Ther. 2008; 15:402–412.
17. Crnkovic-Mertens I, Wagener N, Semzow J, Grone EF, Haferkamp A, Hohenfellner M, et al. Targeted inhibition of Livin resensitizes renal cancer cells towards apoptosis. Cell Mol Life Sci. 2007; 64:1137–1144.
18. Bavykin AS, Korotaeva AA, Poyarkov SV, Syrtsev AV, Tjulandin SA, Karpukhin AV. Double siRNA-targeting of cIAP2 and LIVIN results in synergetic sensitization of HCT-116 cells to oxaliplatin treatment. Onco Targets Ther. 2013; 6:1333–1340.
19. Liu F, Chang H, Xu W, Zhai Y. The effects of Livin shRNA on the response to cisplatin in HepG2 cells. Oncol Lett. 2015; 10:2957–2961.
20. Kasof GM, Gomes BC. Livin, a novel inhibitor of apoptosis protein family member. J Biol Chem. 2001; 276:3238–3246.
21. Wang L, Zhang Q, Liu B, Han M, Shan B. Challenge and promise: roles for Livin in progression and therapy of cancer. Mol Cancer Ther. 2008; 7:3661–3669.
22. Ryther RC, Flynt AS, Phillips JA 3rd, Patton JG. siRNA therapeutics: big potential from small RNAs. Gene Ther. 2005; 12:5–11.
23. Park DG. Antichemosensitizing effect of resveratrol in cotreatment with oxaliplatin in HCT116 colon cancer cell. Ann Surg Treat Res. 2014; 86:68–75.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download