Abstract
Congenital anomalies of the inferior vena cava (IVC) are rare but important problems in living donors for kidney transplantation, especially in cases of a short left renal vein and accompanying vascular and urological anatomic variations. However, the clinical impacts of IVC anomalies in deceased donors have yet to be reported. The unexpected presence of an IVC in an unusual position poses challenges to surgeons and increases the risk of bleeding during organ removal. Accompanying vascular variations can cause unexpected bleeding and injury and therefore technical complications in procurement and subsequent implantation. During cold perfusion, inadequate venous drainage or insufficient cooling can induce graft damage. Our cases highlight the need for all transplant surgeons to confirm the anatomy of the aorta, IVC, and major vessels early in the surgical procedure and, should an anomaly be detected, know how to manage the problem.
Congenital anomalies of the inferior vena cava (IVC) are rare and in most cases are discovered incidentally on imaging studies carried out for other reasons. Therefore, the exact prevalence is not known. Previous reports on IVC anomalies were based on cadaveric dissection studies, large-scale CT studies, and studies of living donors for kidney transplantation [123]. According to their results, the prevalence of IVC anomalies is 0.2%–3%. In general, IVC anomalies do not cause significant problems. However, in living donors for kidney transplantation they can be a deciding factor in selecting the optimal kidney for donation. Nephrectomy procedures for a donor with IVC anomalies differ from those used in donors with a normal IVC. For example, a shorter left than right renal vein will be accompanied by anatomic variations in the vascular and urological systems [45]. While in such cases extra caution may be needed, most studies have reported few problems related to IVC anomalies, because they are usually detected in imaging studies, such as CT angiography, performed prior to surgery. In contrast to living donor surgery, in procurement surgery from a deceased donor surgeons are not usually provided with anatomic information. Thus, when suddenly confronted with a deceased donor with an IVC anomaly they may be ill-prepared to deal with it or be fully aware of its clinical impact. To date, there have been no reports in the literature on the implications or management of IVCs in deceased donors. Here we present two cases of IVC anomalies, a left-sided IVC (L-IVC) and a double IVC (D-IVC), detected during procurement surgery from deceased donors.
A 55-year-old man was pronounced brain dead following an infarction of the left middle cerebral artery territory. As his family chose to donate his organs, organ procurement surgery was performed. During the surgery, we secured the infrarenal aorta to prepare for perfusion catheter cannulation. However, during the dissection of the right side of the aorta, we were unable to find the IVC. A dissection upwards from both iliac veins revealed the IVC from the left side of the aorta (Fig. 1A). The IVC joined the left renal vein and crossed the aorta anteriorly; it then joined the right renal vein and ran upwards along the right side of the aorta. This anomaly resulted in a left renal vein that was shorter than the right one. Both kidneys had a single artery, and no other anatomic anomaly was identified. The left kidney was procured together with the IVC; the left renal vein was extended during a back-table procedure (Fig. 1B). There were no technical difficulties during the surgery and the recipient recovered well from the transplant procedure without delayed graft function or other complications.
A 44-year-old woman was pronounced brain dead following the rupture of a middle cerebral artery aneurysm. Her family chose to donate her organs and procurement surgery was performed. After the infrarenal aorta and the IVC had been located in their normal positions, cold perfusion was performed. Thereafter, the dissection was continued around both renal veins and arteries, which revealed an atypically large vein next to the gonadal vein, with both veins draining into the left renal vein (Fig. 2A). The large vein originated from the left common iliac vein and was identified as a second IVC, located on the left side of the aorta. This left IVC crossed the aorta just after its union with the left renal vein and then joined the right IVC to form a single, right-sided IVC. The right kidney had double renal arteries, but there were no other anatomic variations. Both kidneys were procured en bloc and divided along the middle of each IVC. Both renal veins were extended with the IVC (Fig. 2B). There were no technical difficulties during the procedures. The kidney recipients recovered well from the transplant procedures without delayed graft function or other complication.
Congenital anomalies of the IVC are caused by defects in fusion and regression processes during the development of the embryonic venous system. Between the sixth and eighth weeks of gestation, three pairs of embryonic veins emerge: the subcardinal, supracardinal, and posterior cardinal veins. These 3 veins fuse to form the vena cava, with the right subcardinal vein contributing the suprarenal segment, the subcardinal and right supracardinal veins becoming the renal segment, the right supracardinal vein forming the infrarenal segment, and the 2 posterior cardinal veins becoming the common iliac veins. However, during these steps, regression of the right supracardinal vein, but persistence of the left one, results in L-IVC, whereas persistence of both supracardinal veins with failure of common iliac vein union gives rise to a D-IVC. The L-IVC runs along the left side of the aorta and joins the left renal vein. The 2 veins cross the aorta anteriorly and unite with the right renal vein in L-IVC, or with the right IVC in D-IVC, and then form a single, right-sided IVC [56].
IVC anomalies raise three important clinical issues in living kidney donors. First, a donor with an IVC anomaly will have a short left renal vein [45]. In a L-IVC donor, the right renal vein is longer than the left one, in which case the right kidney is preferred for donation. In a D-IVC donor, both renal veins are short and a venous anastomosis to the external iliac vein may be insufficient to extend either one. Among the methods used by other authors to overcome this problem were: ligation and division of the internal iliac vein to mobilize the external iliac vein, elongation of the renal vein using autologous vein or artificial vascular graft [47] or ligation and division of the left IVC and taking the left kidney, with a sufficient length of renal vein [7]. In the third of these three methods, however, swelling of the scrotum or of the ipsilateral lower extremity is possible as complications. In a donor with a short renal vein, we use three methods either alone or in combination: (1) Small branches of the renal vein are ligated and divided during a back-table procedure, which extends the renal vein up to 8 mm without venous congestion. (2) The external iliac vein is then mobilized and the internal iliac vein is ligated and divided; alternatively, a venous anastomosis is made to the common iliac vein or IVC. (3) The renal vein is elongated using the donor's ipsilateral gonadal vein, which lengthens the renal vein by 5–7 mm.
Second, an IVC anomaly is often accompanied by vascular anomalies, such that unexpected bleeding or injury is possible [35]. Therefore, in these donors both the nephrectomy and the implantation are technically difficult. A left IVC can be injured during nephrectomy, by mistaking it for a gonadal vein, as both drain into the left renal vein. In patients with pelvic congestion, the gonadal veins are extremely dilated, which increases the likelihood that the left IVC will be misidentified as a dilated gonadal vein. A D-IVC often occurs together with common iliac vein union failure and unusual interiliac veins will be present between the separated common iliac veins. [3] Other abnormal vessels in an unusual site—caused by regression failure during development—can induce unexpected bleeding. Multiple renal arteries and veins or a retroaortic or circumaortic renal vein are common variations found together with IVC anomalies and increase the risk of injury or bleeding [26].
Third, urological anomalies or a horseshoe kidney are common in donors with IVC anomalies and will need to be carefully inspected during the preoperative evaluation or during surgery. The prevalence of IVC anomalies is 0.2%–3% in the general population, but 5.7% among individuals with a horseshoe kidney [8]. In donors from the latter group, the venous anatomy must be examined. The most common form of urological anomaly is a retrocaval ureter [6]. In L-IVC or D-IVC, the infrarenal IVC originates from the left posterior cardinal vein, such that the IVC is located on the anteriolateral side of the left ureter and thus causes proximal ureter obstruction. A retrocaval ureter will induce hydronephrosis with or without urinary tract infection [6].
Several groups have reported venous hypertension in L-IVC or D-IVC due to inadequate venous drainage, leading to venous stasis and deep vein thrombosis (DVT). Previous studies have shown an increase in DVTs of the lower extremities in patients with IVC anomalies [9].
Yang et al. [10] reported nutcracker syndrome in a patient with L-IVC, in which the IVC crosses the aorta anteriorly and joins the right renal vein. During this course, if the IVC meets a superior mesenteric artery (SMA) that has emerged at an angle of <30°, the IVC will be compressed by the SMA such that both the IVC and the left renal vein are dilated and venous hypertension is induced. The patient described in that report had an initial venous pressure in the left renal vein of 20 cmH2O, which decreased to 5 cmH2O after decompression by SMA transposition.
Some of the above-discussed issues also occur in deceased donors with IVC anomalies, but there are also several differences regarding their clinical importance and additional issues that have to be considered. Procurement surgery in a deceased donor has a greater risk of injury or bleeding than surgery in a living donor. In deceased donors, preoperative anatomic information is often lacking because when the donor was alive his or her vital signs were not stable enough to allow preoperative imaging studies. Thus, surgeons will often first be aware of anatomic problems with the donor during surgery.
Studies of IVC anomalies in deceased donors have not been previously reported or mentioned in the literature. The absence of the IVC in its expected location can result in surgical errors and massive bleeding. Moreover, because procurement surgery is frequently performed in emergent situations, with different surgeons dissecting the organs for transplantation in their patients, there is no opportunity for a systemic and comprehensive dissection. Instead, limited and minimal dissections are carried out around anatomic landmarks and any variations may be overlooked.
The clinical impact of an IVC anomaly in a deceased donor differs in several aspects from the same anomaly in a living donor. In the former, renal vein length is not an important problem, as a short renal vein can be extended with the IVC. We used the left kidney from the L-IVC donor and the right kidney from the D-IVC donor without technical difficulties during implantation in the respective recipients.
Accompanying vascular and urological anomalies raise important issues in deceased donor kidney transplantation. In contrast to nephrectomy in a living donor, many parts of the kidney procurement procedure in deceased donors are performed after the completion of cold perfusion. During the cold dissection, vascular injuries are very likely to be overlooked, resulting in injuries to multiple renal arteries or veins. Thus, if an IVC anomaly is detected, attention must be paid to the possibility of vasculature multiplicity, during both procurement and back-table procedures. This information must be supplied to the center that will receive the kidney pair, together with information on the presence of a retrocaval ureter and the suitability of the graft for transplantation.
Finally, there must be sufficient vein drainage during cold perfusion. In a D-IVC, if the presence of a left IVC is overlooked, then venous drainage may be compromised. A left IVC receives a large volume of blood from the lower extremities, as well as perfusate from the kidney. The blood and perfusate mix at the point where the left IVC crosses in front of the aorta, at which point the same mixture of fluids enters from the right IVC. As previous studies of venous hypertension and nutcracker syndrome have reported, a left IVC poses a greater handicap for venous drainage than a normal right-sided IVC [910]. Severe and persistent venous hypertension can be transmitted to the left kidney and cause edema or damage to the renal parenchyma. During further dissection after the completion of cold perfusion, the continuous drainage of blood from the lower extremity through the left IVC can hinder effective cooling of the left kidney. Therefore, in a D-IVC donor, a venotomy for venous drainage should be made in each IVC. In the 2 cases described herein, the SMA was far from the IVC segment crossing the aorta, and the angle of SMA emergence was not acute. Thus, the SMA did not affect the IVC or left IVC, and renal vein dilatation was not observed. In the D-IVC donor, we failed to notice the presence of a left IVC and did not perform a venotomy to allow for venous blood drainage; however, graft function was not compromised.
In conclusion, as congenital anomalies of the IVC are very rare, they are not suspected in deceased donor procurement surgery. However, they can cause unexpected bleeding or injury to the vascular structures of the graft as well as problems during cannulation and perfusion procedures. Therefore, all transplant surgeons should examine the anatomy of the aorta, IVC, and major vessels before cold perfusion and, should anomalies be detected, be well aware of how to manage them.
Figures and Tables
Fig. 1
Kidneys from deceased donor with left-sided inferior vena cava (IVC). (A) Renal grafts after en bloc procurement. Left-sided IVC joined the left renal vein and crossed the aorta anteriorly and then joined the right renal vein. Left renal vein was shorter than the right one. Both kidneys had a single artery. (B) The left kidney was procured together with the IVC. The left renal vein is extended during a back-table procedure using the IVC. L, left-sided IVC; Ao, aorta; SMA, superior mesenteric artery; LK, left kidney.
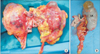
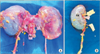
Fig. 2
Kidneys from deceased donor with double inferior vena cava (IVC). (A) Right IVC and second left IVC were identified. Left IVC crossed the aorta just after its union with the left renal vein and then joined the right IVC to form a single, right-sided IVC. The right kidney had double renal arteries (arrow, inferior polar artery arising from aorta) but there were no other anatomic variations. (B) Right kidney after back-table procedure. Right renal vein is extended with IVC. R, right-sided IVC; L, left-sided IVC; Ao, aorta; RK, right kidney.
References
1. Reis RH, Esenther G. Variations in the pattern of renal vessels and their relation to the type of posterior vena cava in man. Am J Anat. 1959; 104:295–318.
2. Aljabri B, MacDonald PS, Satin R, Stein LS, Obrand DI, Steinmetz OK. Incidence of major venous and renal anomalies relevant to aortoiliac surgery as demonstrated by computed tomography. Ann Vasc Surg. 2001; 15:615–618.
3. Morita S, Higuchi M, Saito N, Mitsuhashi N. Pelvic venous variations in patients with congenital inferior vena cava anomalies: classification with computed tomography. Acta Radiol. 2007; 48:974–979.
4. Sakai H, Ide K, Ishiyama K, Onoe T, Tazawa H, Hotta R, et al. Renal vein extension using an autologous renal vein in a living donor with double inferior vena cava: a case report. Transplant Proc. 2012; 44:1446–1449.
5. Ang WC, Doyle T, Stringer MD. Left-sided and duplicate inferior vena cava: a case series and review. Clin Anat. 2013; 26:990–1001.
6. Bass JE, Redwine MD, Kramer LA, Huynh PT, Harris JH Jr. Spectrum of congenital anomalies of the inferior vena cava: cross-sectional imaging findings. Radiographics. 2000; 20:639–652.
7. Davari HR, Malek-Hosseini SA, Salahi H, Bahador A, Nikeghbalian S, Jalaeian H, et al. Management of infrarenal duplicated inferior vena cava during living related kidney transplantation. Transpl Int. 2007; 20:478–479.
8. Ichikawa T, Kawada S, Koizumi J, Endo J, Iino M, Terachi T, et al. Major venous anomalies are frequently associated with horseshoe kidneys. Circ J. 2011; 75:2872–2877.
9. Gayer G, Luboshitz J, Hertz M, Zissin R, Thaler M, Lubetsky A, et al. Congenital anomalies of the inferior vena cava revealed on CT in patients with deep vein thrombosis. AJR Am J Roentgenol. 2003; 180:729–732.
10. Yang BZ, Li Z, Wang ZG. Nutcracker syndrome due to left-sided inferior vena cava compression and treated with superior mesenteric artery transposition. J Vasc Surg. 2012; 56:816–818.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download