Abstract
Purpose
Postoperative pancreatic fistula (POPF) is one of the most common and clinically relevant complications after distal pancreatectomy. Some aspects of POPF management remain controversial. Therefore, the aim of this study was to determine the natural course of POPF and fluid collection after distal pancreatectomy and to reappraise the necessity of intraoperative abdominal drainage insertion.
Methods
For recent 10 years, 264 distal pancreatectomies were performed at Seoul National University Hospital. Clinicopathologic data including POPF and postoperative fluid collection (POFC), and its treatment modality were reviewed retrospectively. During follow-up, the location, size, and clinical impact of the POFC were determined on the basis of CT images.
Results
Clinically relevant POPFs were identified in 72 patients (27.3%). Therapeutic interventions were performed in 40 patients (55.6%), and conservative management was successful in 32 patients (44.4%). POFC was detected in 191 cases (72.3%) on the first postoperative CT. During follow-up, spontaneous regressions were observed in 119 cases (93.0%). Only thick pancreatic stump increased the risk of clinically relevant POPF (≥17.3 mm, P = 0.002) and the occurrence of POFC (≥16.0 mm, P < 0.001) in multivariate analysis.
Postoperative pancreatic fistula (POPF) is one of the most common complications of pancreatic surgery [1]. Recent advances in operative techniques and perioperative care have resulted in lower morbidity and mortality rates; however, POPFs may still lead to intra-abdominal abscess, hemorrhage, and sepsis. Although the early diagnosis and prevention of POPF have been extensively discussed, the management of patients after distal pancreatectomy remains unclear [2].
Given that pancreatic juice is not activated after distal pancreatectomy, the severity and clinical course of the POPF is milder after distal pancreatectomy than after pancreatoduodenectomy [3]; thus, the postoperative management after distal pancreatectomy should be different from that after pancreatoduodenectomy. However, little is known about the optimal management of POPFs including the optimal drainage of the remnant pancreas, the optimal technique for closure of the pancreatic stump, and the clinical impact and natural course of the fluid collection that is commonly observed in the postoperative CT scans.
Therefore, the aim of this study was to evaluate the natural course of the POPF after distal pancreatectomy and to reappraise the necessity of intraoperative abdominal drainage insertion.
A total of 264 distal pancreatectomies were performed by 2 experienced pancreatic surgeons at Seoul National University Hospital from May 2004 to April 2013. Patient characteristics were reviewed for age, sex, histologic diagnosis, operative method, type of stump closure, pancreatic texture, size of the main pancreatic duct, pancreatic thickness, presence of POPF, and treatment modality for POPF. Moreover, the postoperative fluid collection (POFC) including its location, size, and clinical impact was assessed during follow-up on the basis of CT images. Patients with no postoperative follow-up CT scans were excluded from the study.
Pancreatic transection was performed with either 2 types of stapler or with a scalpel and a hand-sewn suture of the pancreatic remnant. The transection level was determined according to tumor location and size, which was classified into the following 4 types in this study: the left side of the gastroduodenal artery, superior mesenteric vein, left border of the aorta, and far distal side of the pancreas.
Reinforcement of closure of the pancreatic stump was performed in the cases with nonstapler closure of the pancreatic remnant, and a very thick pancreas, which are associated with an increased risk of POPF. Fibrin sealant was always applied to the pancreatic stump. TachoSil (Nycomed, Linz, Austria) or Neoveil (Gunze Ltd., Osaka, Japan) was selectively applied to decrease the occurrence of POPF. A surgical drain was always placed intraoperatively. A 10-mm silastic drain was intraoperatively placed and anchored onto the pancreatic stump via the left subphrenic space.
A regular diet was started on postoperative day 2 or 3. Abdominal CT scans were performed on postoperative days 4–7 to exclude postoperative complications such as POFC and to determine whether to remove the surgical drain. The removal of the surgical drain was delayed until the daily amount of fluid decreased below 10 mL in patients with POFC and well-functioning surgical drains or in those without POFC but high drain amylase levels. An interventional procedure such as surgical drain repositioning or insertion of a new percutaneous drainage (PCD) catheter was performed in patients with POFC who had an ineffective surgical drain and associated leukocytosis, symptoms, or fever. Endoscopic aspiration of pseudocyst or cystogastrostomy was performed only when a percutaneous approach was not feasible or had failed. The drain was removed immediately after CT scan in patients without POFC, and those with POFC who did not have any associated clinical impact.
After discharge, a follow-up abdominal CT scan was performed after 3, 6, and 12 months. Additional CT scans were selectively performed 1 month after discharge in patients with POPF, and those with POFC requiring specific treatment.
The diagnosis of pancreatic fistula was determined according to the criteria established by the International Study Group on Pancreatic Fistula (ISGPF) [4]. Pancreatic fistulas are classified by the ISGPF and the Clavien-Dindo classification [5]. In patients with peripancreatic fluid collection and an ineffective drain making the drain amylase levels unreliable, the ISGPF criteria were not applicable. Therefore, these patients were classified as having POFC without evidence of POPF, and their clinical course was investigated.
Contrast-enhanced, dynamic multidetector CT studies with a 3-mm thickness interval were performed preoperatively and postoperatively. The size of the main pancreatic duct and the pancreatic thickness of the transection site were measured preoperatively on axial CT images during the late arterial phase. The transection line was estimated based on postoperative CT images. The size of POFC was defined as the longest diameter of the peripancreatic fluid collection based on the postoperative CT images at 7 days, and at 1, 3, 6, and 12 months, in order to determine the POFC changes.
Statistical analysis was performed with IBM SPSS Statistics ver. 19.0 (IBM Co., Armonk, NY, USA). The chi-square test was used to analyze differences between groups. Univariate and multivariate logistic regression analyses were used to identify predictive factors for the development of POPF and POFC. A P-value of <0.05 was considered statistically significant.
Among the 264 patients with a mean age of 55.8 ± 13.7 years, cystic neoplasms were the most common (n = 130), followed by pancreatic cancer (n = 83), and neuroendocrine tumors (n = 34). Laparoscopy was performed in 106 cases (Table 1). The following complications other than POPF were detected in 11 patients: pleural effusion (n = 3), wound complication (n = 2), pneumonia (n = 2), deep vein thrombosis (n = 2), acute pyelonephritis (n = 1), and immediate postoperative bleeding (n = 1).
Clinically relevant POPFs were identified in 72 patients (27.3%). A surgical drain was placed and anchored in all patients intraoperatively; however, only 33 of the drains remained on the pancreatic stump to ensure effective drainage of the POPF or POFC. Antibiotics were used in 46 patients (17.4%). PCDs were performed in 36 cases (13.6%), as follows: repositioning of a previously inserted surgical drain (n = 8); and insertion of a new catheter (n = 28). Two of the seven cases of endoscopic internal drainage were performed after readmission in the conservatively treated patients, during the initial admission period. The mean hospital stay for all patients was 11.2 ± 7.2 days, and the rate of readmission was 3.8 (Table 2).
There were 4 cases of failure of the percutaneous procedure, representing a failure rate of 10.0. Spontaneous regressions were observed in 2 cases; however, 1 patient underwent endoscopic cystogastrostomy, and the other patient was readmitted for endoscopic insertion of a retrograde pancreatic duct stent, after the initial discharge.
Postoperative CT scans were performed in all patients after 7 days. The follow-up CT scans performed after 1 month (n = 55), 3 months (n = 262), 6 months (n = 245), and 12 months (n = 230) were reviewed. The natural course after distal pancreatectomy is summarized in Fig. 1. The cases of POPF were classified as POPF without POFC (n = 15) and POPF with POFC (n = 74). Ten of the 15 cases of POPF without POFC, prolonged drainage defined as drain placement for ≥10 days were required. A nonsymptomatic, small pseudocyst newly appeared on the pancreatic stump in 10 of these 15 patients during 1-year follow-up; however, none of these patients required additional treatment.
POFC was detected in 191 cases (72.3%). POPF-related POFC and POFC without evidence of POPF were detected in 74 cases (38.7%) and 117 cases (61.3%), respectively. Among the 128 patients (67.0%) who were simply monitored, spontaneous complete regression of the POFC was observed in 119 patients (93.0%). A remnant and silent pseudocyst was found in 7 patients (5.5%).
Seven (11.1%) of the 63 patients who required POFC management including antibiotics, prolonged use of the surgical drain, PCD, and an endoscopic procedure had a remnant silent pseudocyst and did not undergo an additional procedure during the follow-up period.
Of the 206 patients with POPF or POFC, only 2 patients underwent endoscopic cystogastrostomy during follow-up because of postprandial discomfort, while the other 204 patients showed spontaneous regression or a remnant, small, nonsymptomatic pseudocyst at the resection margin (Fig. 1).
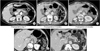
As shown in Fig. 2A-C, fluid collections are initially dispersed around the pancreatic stump. During follow-up, the fluid collection typically became a round-shaped pseudocyst. Among the 63 patients with POFC who required treatment (ISGPF grade B), remnant pseudocysts at the pancreatic stump without any clinical impact were observed in seven cases (Fig. 1). Stapler dislocation (Fig. 2D, E) was observed in 23 (12.0) of 191 patients with POFC.
Clinically relevant POPFs occurred more frequently in patients who underwent procedures with a thicker pancreas transection plane (16.8 mm vs. 19.3 mm, P < 0.001). POFC also occurred more frequently in patients with increased pancreas stump thickness (14.6 mm vs. 18.6 mm P < 0.001), and in those who underwent procedures where the pancreatic transection plane was more distal than the superior mesenteric vein (P = 0.006). There was no difference in the rate of POPF formation or POFC according to the original disease (Tables 3, 4). The receiver operating characteristics (ROC) curve for POPF showed that the area under the ROC curve was greatest at a cutoff value of 17.3 mm. In total, 49.6 had a stump thickness ≥17.3 mm. For POFC, the cutoff value of stump thickness by ROC curve analysis was 16.0 mm, and 161 patients (61.0%) had a stump thickness ≥16.0 mm. Only thick pancreatic stumps increased the risk of clinically relevant POPF (≥17.3 mm; odds ratio [OR], 3.979; P = 0.002) and the occurrence of POFC (≥ 16.0 mm; OR, 7.574; P < 0.001) in multivariate analysis (Table 5).
POPF is one of the most common and clinically relevant complications after pancreatectomy, occurring in 3–34 of cases [6789]. Some aspects of POPF management including the optimal drainage of the remnant pancreas and the technique for closure of the pancreatic stump after distal pancreatectomy remain controversial [2].
With regard to the use of abdominal drains after pancreatoduodenectomy, the use of selective drainage and early drain removal is currently recommended in low-risk patients [1011]. Although a retrospective study reported that drainage did not reduce morbidity or the need for therapeutic intervention [12], and a meta-analysis reported that the routine use of abdominal drains increases the risk of major complications after distal pancreatectomy [131415], there are currently limited data regarding distal pancreatectomy. Moreover, no studies have investigated the long-term radiological changes and clinical impact associated with POPF or POFC after distal pancreatectomy to assist in determining the optimal management of patients after distal pancreatectomy regardless of widely performed postoperative surveillance using imaging modalities. Therefore, serial CT scans were analyzed to evaluate the natural course of the POPF and POFC after distal pancreatectomy in the present study.
The review of the CT images showed that only 33 of the intraoperatively placed abdominal drains remained on the stump of the remnant pancreas despite the efforts to prevent its displacement. Among those, adequate drainage was obtained by prolonged use of surgical drain in 20.7, which is comparable to the previously reported rate of 18 [16]. Among 72 cases of clinically relevant POPF, therapeutic interventions were performed in 40 patients (55.6%) and conservative management was successful in 32 (44.4%).
Several aggressive strategies have been previously reported in terms of the technique used for the closure of the pancreatic stump after distal pancreatectomy to reduce POPF. Preoperative endoscopic sphincterotomy and stenting from a German study [17] were reported as feasible and safe, however, these results were not reproducible in a subsequent randomized controlled trial [18]. Isolated Roux-en-Y anastomosis of the pancreatic stump after distal pancreatectomy [1920], another aggressive strategies adopted in several centers. However, clinical impact of pancreatic occlusion failure after distal pancreatectomy is a milder than that of pancreatic anastomotic failure after pancreatoduodenectomy, because the pancreatic juice is not activated [3]. A previous study reported that cultures were positive in 74 and 31 of fluid collections after pancreatoduodenectomy and distal pancreatectomy, respectively [16]. In this context, several studies report that POPF can be managed conservatively after conventional distal pancreatectomy [921]. The reported success rate of nonoperative management including antibiotics, supplemental nutrition, somatostatin analogues, and adequate drainage ranges from 92 [22] to 95 [21].
Similarly, the most severe complication observed in this study was classified as grade 3A. Of the 128 patients with POFC without management, only 2 patients (1.6%) required therapeutic intervention, while the other 119 patients (93.0%) showed spontaneous regression or a remnant, small, nonsymptomatic pseudocyst at the resection margin which could raise question regarding the necessity of the routine placement of intraoperative abdominal drain.
The only predictive factor of POPF and POFC in this study was a thickness of the pancreatic stump. In patients with a thickness less than 17.3 mm, clinically relevant POPF occurred only in 6 patients (2.3%) among 264 patients. Among the 18 patients with prolonged use of surgical drain, 16 patients had thickness more than 17.3 mm. POFC occurred in 13 cases (4.5%) when thickness was less than 16.0 mm. Therefore, surgical drain could be selectively indwelled in patients with a thick pancreas, however, definitive evidence regarding the necessity and indication usage of surgical drain in the distal pancreatectomy is needed.
This study provides unique information regarding the natural course after distal pancreatectomy based on regular follow-up imaging and clinical data. However, this study is limited in that several types of surgery, transection methods, reinforcement methods of the pancreatic stump were included. Comparison of outcomes between patients with and without a drain, according to POPF risk, was not shown because of the retrospective design of the study and definitive evidence regarding the necessity of surgical drain after distal pancreatectomy is needed. In conclusion, intraoperative abdominal drainage insertion could be selectively indwelled in patients with a thickness of pancreas ≥17.3 mm. Since radiologically-proven POFC after distal pancreatecomy showed a 93.0 rate of spontaneous regression, POFC without signs of infection can be safely monitored.
Figures and Tables
Fig. 1
The natural course of the postoperative pancreatic fistula (POPF) and postoperative fluid collection (POFC) after distal pancreatectomy. PCD, percutaneous drainage; EUS, endoscopic ultrasonography. a)PCD was performed in patients who developed hematomas. b)Clavien-Dindo classification 0 and I. c)Clavien-Dindo classification II–V. d)A "Silent pseudocyst" is defined as a small nonsymptomatic pseudocsyt, which was present at the pancreas resection margin until the end of follow-up.

Fig. 2
Postoperative CT findings after distal pancreatectomy. (A-C) Pseudocyst formation. (A) Postoperative fluid collection (POFC) is initially dispersed around the pancreatic stump. (B, C) The fluid collection typically became a round-shaped pseudocyst during follow-up. (D-E) Stapler dislocation. Stapler dislocation (arrows) was observed in 23 (12.0%) of 191 patients with POFC.
ACKNOWLEDGEMENTs
This study was supported by the Seoul National University Hospital, Republic of Korea (grant no. 0420143010).
References
1. Butturini G, Marcucci S, Molinari E, Mascetta G, Landoni L, Crippa S, et al. Complications after pancreaticoduodenectomy: the problem of current definitions. J Hepatobiliary Pancreat Surg. 2006; 13:207–211.
2. Melloul E, Raptis DA, Clavien PA, Lesurtel M. European-African Hepato-Pancreato-Biliary Association. Poor level of agreement on the management of postoperative pancreatic fistula: results of an international survey. HPB (Oxford). 2013; 15:307–314.
3. Strasberg SM, Linehan DC, Clavien PA, Barkun JS. Proposal for definition and severity grading of pancreatic anastomosis failure and pancreatic occlusion failure. Surgery. 2007; 141:420–426.
4. Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005; 138:8–13.
5. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004; 240:205–213.
6. Kleeff J, Diener MK, Z'graggen K, Hinz U, Wagner M, Bachmann J, et al. Distal pancreatectomy: risk factors for surgical failure in 302 consecutive cases. Ann Surg. 2007; 245:573–582.
7. Balcom JH 4th, Rattner DW, Warshaw AL, Chang Y, Fernandez-del Castillo C. Ten-year experience with 733 pancreatic resections: changing indications, older patients, and decreasing length of hospitalization. Arch Surg. 2001; 136:391–398.
8. Lillemoe KD, Kaushal S, Cameron JL, Sohn TA, Pitt HA, Yeo CJ. Distal pancreatectomy: indications and outcomes in 235 patients. Ann Surg. 1999; 229:693–698.
9. Balzano G, Zerbi A, Cristallo M, Di Carlo V. The unsolved problem of fistula after left pancreatectomy: the benefit of cautious drain management. J Gastrointest Surg. 2005; 9:837–842.
10. Kaminsky PM, Mezhir JJ. Intraperitoneal drainage after pancreatic resection: a review of the evidence. J Surg Res. 2013; 184:925–930.
11. Williamsson C, Karlsson N, Sturesson C, Lindell G, Andersson R, Tingstedt B. Impact of a fast-track surgery programme for pancreaticoduodenectomy. Br J Surg. 2015; 102:1133–1141.
12. Paulus EM, Zarzaur BL, Behrman SW. Routine peritoneal drainage of the surgical bed after elective distal pancreatectomy: is it necessary. Am J Surg. 2012; 204:422–427.
13. van der Wilt AA, Coolsen MM, de Hingh IH, van der Wilt GJ, Groenewoud H, Dejong CH, et al. To drain or not to drain: a cumulative meta-analysis of the use of routine abdominal drains after pancreatic resection. HPB (Oxford). 2013; 15:337–344.
14. Conlon KC, Labow D, Leung D, Smith A, Jarnagin W, Coit DG, et al. Prospective randomized clinical trial of the value of intraperitoneal drainage after pancreatic resection. Ann Surg. 2001; 234:487–493.
15. Fisher WE, Hodges SE, Silberfein EJ, Artinyan A, Ahern CH, Jo E, et al. Pancreatic resection without routine intraperitoneal drainage. HPB (Oxford). 2011; 13:503–510.
16. Vin Y, Sima CS, Getrajdman GI, Brown KT, Covey A, Brennan MF, et al. Management and outcomes of postpancreatectomy fistula, leak, and abscess: results of 908 patients resected at a single institution between 2000 and 2005. J Am Coll Surg. 2008; 207:490–498.
17. Rieder B, Krampulz D, Adolf J, Pfeiffer A. Endoscopic pancreatic sphincterotomy and stenting for preoperative prophylaxis of pancreatic fistula after distal pancreatectomy. Gastrointest Endosc. 2010; 72:536–542.
18. Frozanpor F, Lundell L, Segersvard R, Arnelo U. The effect of prophylactic transpapillary pancreatic stent insertion on clinically significant leak rate following distal pancreatectomy: results of a prospective controlled clinical trial. Ann Surg. 2012; 255:1032–1036.
19. Wagner M, Gloor B, Ambuhl M, Worni M, Lutz JA, Angst E, et al. Roux-en-Y drainage of the pancreatic stump decreases pancreatic fistula after distal pancreatic resection. J Gastrointest Surg. 2007; 11:303–308.
20. Okada K, Kawai M, Tani M, Hirono S, Miyazawa M, Shimizu A, et al. Isolated Roux-en-Y anastomosis of the pancreatic stump in a duct-to-mucosa fashion in patients with distal pancreatectomy with en-bloc celiac axis resection. J Hepatobiliary Pancreat Sci. 2014; 21:193–198.
21. Pannegeon V, Pessaux P, Sauvanet A, Vullierme MP, Kianmanesh R, Belghiti J. Pancreatic fistula after distal pancreatectomy: predictive risk factors and value of conservative treatment. Arch Surg. 2006; 141:1071–1076.
22. Goh BK, Tan YM, Chung YF, Cheow PC, Ong HS, Chan WH, et al. Critical appraisal of 232 consecutive distal pancreatectomies with emphasis on risk factors, outcome, and management of the postoperative pancreatic fistula: a 21-year experience at a single institution. Arch Surg. 2008; 143:956–965.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download