Abstract
Alimentary tract duplications are uncommon congenital anomalies that usually present during the first decade of life. Complete duplication of the colon in adults is very rare and difficult to diagnose preoperatively. We report a case of a 40-year-old female with complete tubular duplication which was initially misdiagnosed as a salpingeal abscess due to colovaginal fistula.
Alimentary tract duplications are uncommon congenital anomalies that usually present during the first decade of life as an acute abdomen or bowel obstruction [1]. Among all duplications, colonic duplication is more rare and complete duplication of the colon in adults is very rare and difficult to diagnose preoperatively. Only few cases have been reported in the literature [234]. In children, several cases of duplication of pouch colon associated with duplication of the lower genitourinary tract have been reported [5]. However, these case reports are extremely rare in adults [6].
Herein, we report a case of complete tubular duplication of the colon in an adult which was initially misdiagnosed as a salpingeal abscess due to pelvic inflammatory disease. To the best of our knowledge, this is a very rare case of a complete tubular colonic duplication presenting as a colovaginal fistula in an adult.
A 40-year-old woman was referred to the Department of Obstetrics and Gynecology with symptoms of chronic pelvic pain since one month and foul smelling vaginal discharge since 2 years. She had no medical history such as constipation and hematochezia. On physical examination, the patient was afebrile with normal vital signs and mild tenderness was present in her lower abdomen without any palpable mass. Transvaginal examination showed normal appearance of the anus and external genitalia, but vaginal discharge was suspected with fecal material. Transvaginal ultrasonogram revealed a normal uterus and both ovaries. Blood Test demonstrated elevated CRP (37 U/L [normal, <1 U/L]), and other serologic tests were within normal limits. Urine analysis showed pyuria (WBCS, 20–29/high power field; leukocytes, 3+) and proteinuria (1+). CT revealed inflammatory change around the left side of the colon and left ovary with loculated fluid collection in the pelvic cavity (Fig. 1). The initial diagnosis was thought to be an abscess in the left salpinx due to pelvic inflammatory disease, and pelviscopic exploration was planned in the gynecologic department. During the operation, no abscess in the pelvic cavity and 2 appendices were observed with inflammatory adhesion among the sigmoid colon and the left salpinx (Fig. 2). When the patient was referred to our department because of severe adhesion and inflammation, we converted her to open surgery. In operative findings, a near total duplicated colon including a duplicated terminal ileum measuring 3 cm in length was observed. It originated from 5 cm above the native ileocecal valve perpendicularly, was parallel to the native ascending, transverse, and descending colon, and terminated in the native proximal sigmoid colon with adherence to the left salpinx. The diameter of the duplicated colon narrowed distally (Fig 3A). When adhesiolysis was performed, fistula formation was observed between the distal end of the duplicated descending colon and the posterior vaginal wall (Fig. 3B). Fistulogram was performed by administration of indigocarmine, and the distal end of the fistula was connected 2 cm above the vaginal orifice to the vaginal posterior wall (Fig. 3C). Blood supply to the duplicated bowel was derived from native ileocecal vessels. These vessels were independent vascular structures without mesentery, and they were observed on the inferior border of the duplicated colon. The duplicated colon showed severe inflammatory change at 15-cm length. And the distal end of the duplicated colon was just underneath the communication with the native sigmoid colon and adhesion to the left salpinx (Fig. 4). Both ends of the duplicated bowel from the duplicated terminal ileum to the duplicated descending colon were resected by using GIA 60 (Tyco Healthcare, Norwalk, CT, USA) (Fig. 3D). Incidental appendectomy with left salpingectomy was also performed. The closure of vaginal opening was not performed. In the pathologic report, the specimen measured 33.0 cm in length. The mucosa of the duplicated ascending and transverse colon was normal. But the lumen of the duplicated descending colon showed a focal stricture with chronic inflammation and a hamartomatous polyp (4 cm × 1.0 cm) (Fig. 5). Her post-operative course was uneventful and she was discharged on the 10th day after operation. She did not require any more treatment, and she has been doing well without any complaints during a follow-up period of 5 years.
Since alimentary tract duplication was first reported by Calder in 1733, congenital cystic abnormalities of the alimentary tract have been assigned several different names such as enterogenous or enteric cysts, giant diverticulum, unusual Meckel's diverticulum, ileum duplex and colon duplex by different authors [1]. They are rare anomalies, detected in 1 of every 4,500 autopsies, and a study in the largest number of cases analyzed 96 infants and children with 101 gastrointestinal (GI) duplications [7]. The most common site of GI duplication is the ileum (30%), followed by the ileocecal valve (30%), jejunum (8%), colon (6%–7%), and rectum (5%) [3]. The anatomical types of GI duplications include the cystic type (90%) and the tubular type (10%). The tubular type includes the double-barreled type (80%) and the Y- or T-shaped type (20%) [8]. Therefore, tubular colonic duplications in adults are very rare, and in our literature review, only 17 cases have been reported [4]. Tubular and Y-shaped total colonic duplications in adults are extremely rare, and we have not found any other cases.
The clinical presentations are related to the location and size of the duplication, the presence of gastric mucosa or pancreatic tissue, and the anatomical type such as cystic or tubular type [9]. These anomalies are frequently encountered during laparotomy for other expected common diseases such as intussusception, appendicitis, GI bleeding, intestinal perforation and obstruction. Therefore, more than 67% of GI duplications are diagnosed within the first year of life [9]. However, if GI duplication is not accompanied by other anomalies such as imperforated anus, perineal abscess, and genitourinary malformation, it is occasionally diagnosed in adulthood because of silent symptoms [3]. Tubular type of colonic duplication has only one connection with the native bowel, and the other side is usually formed by blind ending pouch, or perianal and genitourinary fistula, and imperforated anus [4]. In our case, the colonic duplication was related to the colovaginal fistula, which had a connection between the narrow end of the distal duplicated bowel and the posterior vaginal wall. Because the distal end of the colonic duplication was too narrow to pass feces adequately for decades, it presented as asymptomatic. However, gradually, chronic inflammation and recurrent obstruction of the fistula tract developed, and this situation resulted in episodes of feces passing into the vagina.
GI tract duplications including colonic duplications are difficult to diagnose preoperatively regardless of their location. A radiologic study including a plain abdominal film, ultrasonography and CT can be helpful in detecting the cystic lesion of colonic duplication [10], but it is difficult to determine other conditions such as a small bowel mass, pancreas tumor and Meckel`s diverticulum. Technetium-99m scintigraphy can be used to detect heterotopic gastric mucosa in cases of bleeding [9]. Colonoscopy is a very useful diagnostic tool for double-barreled type and cystic type of colonic duplication [4]. However, in case of tubular type, it may not be diagnostic because in such cases, the distal orifice of the duplicated colon is very small or absent and it may appear similar to the diverticulum. In our case, CT showed that an abscess or mass was located in the left ovary and salpinx; hence, it was misdiagnosed as a tubo-ovarian abscess. If colonoscopy had been performed in our case, there may have been no remarkable findings because proximal ending of the duplicated bowel was the duplicated terminal ileum and its distal ending was fused to the sigmoid colon by fistula formation.
Surgical resection is the standard treatment option for GI duplication in order to avoid possible complications such as obstruction, bleeding, intussusception, perforation and malignant change [4]. Cystic type of GI duplication can be resected completely. If the lesion is located on the mesenteric side, combined resection of the native bowel is recommended so as to avoid injury to the mesenteric blood vessels [4]. In double-barreled type of GI duplication, the long segment is mostly involved; hence, complete resection can cause short bowel syndrome. Therefore, in such cases, selective mucosal stripping can be a successful treatment option. It is a good method that helps to avoid extensive bowel resection and ensures removal of ectopic mucosa [9]. In tubular type of GI duplication such as in our case, it can be resected completely in most cases, and in these cases, both the native and duplicated bowel often share the blood supply, and therefore, it is important that ligation of blood vessels to the duplicated bowel should be performed close to the duplicated bowel wall [4]. When combined resection including the native bowel is needed, the range of surgical resection should not go beyond to eliminate the patient's symptoms [9]. Although malignant change in the duplicated bowel has rarely been reported in adulthood, resolution of symptoms and prevention of recurrence are thought to be more important for retaining the normal anatomy than extensive resection [10]. Our case is of a nearly total colonic duplication with marginal vessels which originated from the branch of the ileocecal artery. Most of the duplicated bowel was resected and ligation of blood vessels was done near to the proximal duplicated ileal wall. But between the sigmoid colon and the upper rectum as though it is double barrel type of duplication were not resected. The diameter of the distal duplicated colon was very narrow and it nearly obstructed the fistula; hence, it was thought to be enough for relieving the vaginal discharge.
In conclusion, total colonic duplication in adulthood is extremely rare and it can often be misdiagnosed due to unexpected symptoms such as abdominal mass, constipation, and vaginal discharge. Therefore, when a duplicated bowel is found incidentally on diagnostic laparotomy due to different diagnosis for another disease, and when the patient complains of nonspecific symptoms including chronic abdominal pain, constipation, palpable mass, even if there is perianal, urinary, vaginal discharge in adults and when radiologic findings reveal an intraabdominal mass attached to the bowel wall, GI duplication should always be considered, and the surgical procedure should be performed adequately according to the type of duplication.
Figures and Tables
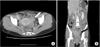 | Fig. 1CT findings. Inflammatory change around the left side of the colon and left ovary (white [A] and black [B] arrow) with loculated fluid collection in the A B pelvic cavity. |
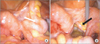 | Fig. 2Laparoscopy showing 2 appendices (white [A] and black [B] arrow) with inflammatory adhesion between the sigmoid colon and the left salpinx. |
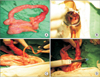 | Fig. 3(A) Tubular duplication of the total colon consisting of the cecum, ascending, transverse, descending colon and the narrowed portion. (B) The distal end of the fistula was connected to the vaginal posterior wall. (C) Fistulogram with indigocarmine. (D) Both ends of the duplicated bowel were resected by using GIA 60 (Tyco Healthcare, Norwalk, CT, USA). |
References
1. Ildstad ST, Tollerud DJ, Weiss RG, Ryan DP, McGowan MA, Martin LW. Duplications of the alimentary tract. Clinical characteristics, preferred treatment, and associated malformations. Ann Surg. 1988; 208:184–189.
2. Caklili OT, Tuncer I, Colak Y, Kosemetin D, Ceyran AB. Colonic duplication in adulthood presenting with diarrhea. Endoscopy. 2013; 45:Suppl 2 UCTN. E430–E431.
3. Banchini F, Delfanti R, Begnini E, Tripodi MC, Capelli P. Duplication of the transverse colon in an adult: case report and review. World J Gastroenterol. 2013; 19:586–589.
4. Yong YG, Jung KU, Cho YB, Yun SH, Kim HC, Lee WY, et al. Large tubular colonic duplication in an adult treated with a small midline incision. J Korean Surg Soc. 2012; 82:190–194.
5. Sarin YK, Manchanda V, Sharma A, Singhal A. Triplication of colon with diphallus and complete duplication of bladder and urethra. J Pediatr Surg. 2006; 41:1924–1926.
6. Shah KR, Joshi A. Complete genitourinary and colonic duplication: a rare presentation in an adult patient. J Ultrasound Med. 2006; 25:407–411.
7. Holcomb GW 3rd, Gheissari A, O'Neill JA Jr, Shorter NA, Bishop HC. Surgical management of alimentary tract duplications. Ann Surg. 1989; 209:167–174.
8. Kokoska ER, Steinhardt GF, Tomita SS, Weber TR. Prostatorectal fistula associated with tubular colorectal duplication. J Pediatr Surg. 1999; 34:1546–1548.
9. Kim TW, Jung PM. A clinical study of intestinal duplication. J Korean Assoc Pediatr Surg. 2004; 10:9–16.
10. Kwak JM, Boo YJ, Kim J. Tubular colorectal duplication presenting as rectovaginal fistula. ANZ J Surg. 2014; 84:289–290.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download