Abstract
Purpose
To evaluate the feasibility of transanal total mesorectal excision (TME) in patients with rectal cancer.
Methods
This study enrolled 12 patients with clinically node negative rectal cancer located 4–12 cm from the anal verge who underwent transanal endoscopic TME with the assistance of single port laparoscopic surgery between September 2013 and August 2014. The primary endpoint was TME quality; secondary endpoints included number of harvested lymph nodes and postoperative complications within 30 days (NCT01938027).
Results
The 12 patients included 7 males and 5 females, of median age 59 years and median body mass index 24.2 kg/m2. Tumors were located on average 6.7 cm from the anal verge. Four patients (33.3%) received preoperative chemoradiotherapy. Median operating time was 195 minutes and median blood loss was 50 mL. There were no intraoperative complications and no conversions to open surgery. TME was complete or nearly complete in 11 patients (91.7%). Median distal resection and circumferential resection margins were 18.5 mm and 10 mm, respectively. Median number of harvested lymph nodes was 15. Median length of hospital stay was 9 days. There were no postoperative deaths. Six patients experienced minor postoperative complications, including urinary dysfunction in 2, transient ileus in 3, and wound abscess in 1.
Conclusion
This pilot study showed that high-quality TME was possible in most patients without serious complications. Transanal TME for patients with rectal cancer may be feasible and safe, but further investigations are necessary to evaluate its long-term functional and oncologic outcomes and to clarify its indications.
Total mesorectal excision (TME) has become the standard surgical method for rectal cancers, reducing local recurrence rates and increasing overall survival [1]. Advances in laparoscopic surgery have resulted in laparoscopic TME replacing traditional open TME, with laparoscopic rectal cancer surgery yielding better short-term outcomes and similar long-term oncologic outcomes compared with open surgery [234]. Laparoscopic TME, however, remains technically demanding in patients with a narrow pelvis, bulky tumor, or obesity, resulting in a high open conversion rate of up to 34% [2]. These challenges have been overcome by the development of the transanal approach, which has been shown to be feasible in patients with rectal cancer [5678910].
The transanal approach has been shown to avoid the need for a permanent colostomy in patients with low-lying rectal cancer after preoperative chemoradiotherapy [11]. Initially, a full-thickness circumferential incision is made at the dentate line, ensuring a sufficient distal margin to the tumor and sparing the anal sphincter muscle. When first introduced, however, this procedure did not use laparoscopic instruments or a transanal platform [11].
The conventional laparoscopic approach has led to the development of natural orifice transluminal endoscopic surgery (NOTES) for TME, frequently involving the transanal route [6]. The first reported transanal TME in a human patient using a transanal endoscopic microsurgery (TEM) platform involved transabdominal laparoscopic assistance [6]. Because NOTES procedures have many technical challenges, including reliable closure of the visceral opening, triangulation of instruments, and stable tissue retraction, these methods must be evaluated thoroughly before NOTES becomes universally accepted as standard clinical practice [8]. This pilot study was therefore designed to evaluate the safety and feasibility of transanal TME in patients with rectal cancer, including an evaluation of technical requirements and pitfalls that may be encountered with this technique.
This study enrolled 12 patients with biopsy-proven rectal adenocarcinoma who underwent transanal TME from September 2013 to August 2014 at the National Cancer Center, Goyang, South Korea. All investigations were conducted according to the principles in the Declaration of Helsinki. The ethics committee of the National Cancer Center approved this study (NCCCTS-13681), and the protocol was registered at clinicaltrial. gov (NCT01938027).
All patients underwent standard tumor staging, including digital rectal examination; flexible endoscopy; pelvic MRI; CT of the chest, abdomen and pelvis; and measurement of serum concentrations of CEA level. Eligible patients were aged 20 to 80 years, with clinically staged T1, T2, and T3 node-negative rectal adenocarcinomas located 4–12 cm from the anal verge without involvement of mesorectal fascia on pelvic MRI, and no evidence of distant metastasis. Patients with T4 tumors, inflammatory bowel disease, previous colorectal malignancy, or extensive prior abdominal or pelvic surgery were excluded. Pregnant women, patients categorized as American Society of Anesthesiologists class 3 or 4 or with an Eastern Cooperative Oncology Group score greater than 2, or those with a body mass index (BMI) greater than 30 kg/m2 were also excluded. Patients with T3 tumors underwent standard preoperative external beam radiotherapy with 50.4 Gy and 5-fluorouracil based concurrent chemotherapy, if indicated. Surgery was performed 6–8 weeks after the end of the neoadjuvant treatment. Patients received postoperative 5-fluorouracil based adjuvant chemotherapy for 4 months. A protective loop ileostomy was constructed as decided by each individual surgeon, and intestinal continuity was re-established after completion of adjuvant chemotherapy. Diet was resumed as soon as the first flatus had been passed. Anal sphincter function was evaluated by the Wexner fecal incontinence score preoperatively and 3 months and 1 year postoperatively [12]. In patients who underwent diverting ileostomy, anal sphincter function was evaluated after stoma repair.
The quality of the mesorectum was scored using three grades: complete, nearly complete, and incomplete [13]. Complete was defined as an intact mesorectum with minor irregularities of a smooth mesorectal surface; no defect was deeper than 5 mm, there was no coning toward the distal margin of the specimen, and slicing showed a smooth circumferential resection margin. Nearly complete was defined as moderate bulk to the mesorectum, but irregularity of the mesorectal surface; moderate coning of the specimen was allowed; and the muscularis propria was not visible at any site, except for the sites of insertion of the levator muscles. Incomplete was defined as little bulk to the mesorectum with defects extending onto the muscularis propria and/or very irregular circumferential resection margins.
The circumferential resection margin was considered positive when the distance from the tumor to the margin was ≤1 mm [14]. After surgery, tumor stage was assessed according to the TNM classification system. Additionally, the maximum diameter, length and location of the tumor were recorded.
The primary endpoint was TME quality. Secondary endpoints were number of harvested lymph nodes and 30-day postoperative complications. Patient and tumor characteristics, operative findings, and length of hospital stay were also recorded. Quantitative data were reported as medians and ranges.
All patients underwent bowel preparation and received prophylactic antibiotics. Under general anesthesia, patients were placed in the lithotomy position for both the abdominal and transanal procedures. To ensure the safe and efficient completion of both, 2 laparoscopic systems were used, each with its own insufflator, camera and monitor. In all cases, transabdominal and transanal approaches were performed simultaneously by two colorectal surgeons.
The abdominal procedure was performed laparoscopically using a single port device (Octoport, Dalim, Seoul, Korea) on the potential ileostomy site of the right iliac fossa, with or without one or 2 additional ports on the left side of the abdomen, which is usually used as the site of intraperitoneal drainage (Fig. 1). In all cases, a rigid 30 degree, 10-mm camera was used. Pneumo-peritoneum was created using a standard laparoscopic insufflator at a pressure of 12–14 mmHg, with 30 L/min CO2 flow.
Low ligation of the inferior mesenteric vessels (IMA) was performed while thoroughly clearing all lymph nodes at the base of the IMA and preserving the ascending colic branch. Mesorectal dissection was performed, taking care to preserve the autonomic nerve, until the level of the Douglas pouch was reached, where the abdominal dissection usually met the transanal procedure. The splenic flexure was mobilized when bowel length was not sufficient for anastomosis.
The perineal region was prepped and draped, and the rectum thoroughly rinsed with a betadine solution. After a digital rectal examination and after confirming the location of the rectal tumor, the anal sphincter was gently dilated manually. A Scott retractor (Lone Star Medical Products, Houston, TX, USA) was inserted and left in place for the duration of the procedure. This retractor facilitated the full-thickness circumferential transection and insertion of a GelPOINT path platform (Applied Medical, Rancho Santa Margarita, CA, USA) (Fig. 1).
If the tumor was located within 2–3 cm from the anorectal ring, a full-thickness circumferential rectal incision was made, followed by dissection to free up 2–3 cm from the anorectal ring. These procedures were usually performed under direct vision because the GelPOINT path for transanal rectal dissection requires fixing the sleeve portion to the anal canal. After secure closure of the rectal stump with 2-0 Prolene purse-string sutures, the surgical field was irrigated with povidoneiodine solution and normal saline to prevent the implantation of cancer cells and infection.
For more proximal tumors, a GelPOINT path was introduced into the anal canal immediately prior to beginning the transanal minimally invasive procedure. After marking by electric diathermy just distal to the suture closure, tight 2-0 Prolene purse-string sutures were placed within the rectum to close the lumen distal to the tumor. A pneumorectum or pneumoperirectum was created using a standard laparoscopic insufflator at a pressure of 6–10 mmHg and a CO2 flow of 5–10 L/min. A standard flexible 10-mm camera and conventional laparoscopic instruments were used.
Posterior rectal dissection was usually performed initially. After sufficient dissection of the posterior aspect, the dissection was maintained anteriorly, then circumferentially, to the lateral part to identify the pelvic autonomic nerve. The neurovascular bundles were identified at the 2 and 10 o'clock positions during anterolateral dissection, during which bleeding and consequent nerve injury were anticipated.
After complete pelvic dissection, the resected specimens, including the tumors, were extracted through a single abdominal port. Depending on the level of the distal rectal incision, a colorectal or coloanal anastomosis was made. When the distal rectal transection was made in the anal canal or near the anorectal ring, a hand-sewn coloanal or colorectal anastomosis was performed. When the distal transection was more proximal, double purse-string sutures were placed in the proximal and distal bowels, allowing a single-stapled anastomosis to be made using a circular stapler [15]. Because mobilization of the distal rectum is technically demanding when using the transanal approach, it is difficult to utilize full-thickness pursue-string sutures in the distal rectum [14]. Therefore, if indicated, additional distal rectal dissections were performed in the abdominal phase, while inserting a spike of the shaft for stapled anastomosis through the center of the purse-string suture.
Between September 2013 and August 2014, 12 patients (7 males and 5 females) underwent transanal endoscopic TME with laparoscopic assistance. Patient demographic and tumor characteristics are summarized in Table 1. Median patient age was 59 years (range, 49–77 years) and median BMI was 24.2 kg/m2 (range, 21.2–29.8 kg/m2). All tumors were located 4 to 8 cm from the anal verge, except one located 12 cm from the anal verge. Four patients with T3 tumors completed standard preoperative chemoradiotherapy.
Intraoperative findings are summarized in Table 2. Laparoscopic procedures were performed using a single port device with an additional one or two 5-mm trocars in 7 patients and additional 12-mm trocars in 3 patients. Two patients underwent laparoscopic procedures using only a single port device without any additional port.
The median operating time in the 12 patients was 195 minutes (range, 105–234 minutes) and median blood loss was 50 mL (range, 0–450 mL). Median time for low ligation of the inferior mesenteric artery was 42 minutes (range, 27–75 minutes). Median time for communication between transanal and abdominal dissection was 78 minutes (range, 52–183 minutes), and median time for specimen removal was 145 minutes (range, 79–201 minutes). There were no intraoperative complications or conversions. The IMA pedicle was transected laparoscopically in all patients, with a diverting ileostomy created in 10 (83.3%).
Postoperative outcomes are summarized in Table 3. Median time to first flatus was 2 days (range, 1–6 days) and median overall hospital stay was 9 days (range, 8–21 days). Six patients experienced postoperative complications, including 3 with ileus, 2 with urinary dysfunction, and 1 with wound infection at the stoma site. All 6 patients were managed conservatively and all fully recovered within 3 weeks after the operation. There were no postoperative deaths.
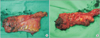
Pathologic outcomes are summarized in Table 4. The median specimen length was 13.6 cm (range, 12.5–26 cm), and the median long tumor diameter was 1.9 cm (range, 0–3.2 cm). Six lesions were anterior or anterolateral, 3 were posterior or posterolateral and 3 were lateral. The TME quality of specimens was complete in 9 patients (75.0%), nearly complete in 2 (16.7%) and incomplete in 1 (8.3%) (Fig. 2). Distal resection margins (DRM) were greater than 5 mm in all patients, with a median DRM of 18.5 mm (range, 6–97 mm). Circumferential margins were greater than 1 mm in 11 patients (91.7%) defining them as negative. The median circumferential margin was 10 mm (range, 1–17 mm), and the median number of harvested lymph nodes was 15 (range, 9–21). More than 12 lymph nodes were harvested from each of 10 patients (83.3%).
An ileostomy was constructed in 10 patients (83.3%); of these, 8 underwent ileostomy closure within 4 months after transanal TME. The remaining 2 patients underwent closure within 1 year. At a median follow-up of 19 months (range, 14–25 months), none of these patients showed evidence of recurrence.
None of the 12 patients reported preoperative fecal incontinence. At a median follow-up of 17 months (range, 6–23 months) after restoring bowel continuity, continence was intact in 8 patients (66.6%), 2 (16.7%) had mild fecal incontinence. and 2 (16.7%) had moderate to severe fecal incontinence, resulting in lifestyle modifications. These 2 patients had Wexner fecal incontinence scores of 8 and 14, respectively, whereas the other 10 had scores ranging from 0–2.
The present pilot study was designed to assess the feasibility and safety of transanal TME for rectal cancers, including the adequacy of oncologic resection and perioperative outcomes, prior to initiating a larger phase II study. This study found that complete or nearly complete TME was achieved in 11 of 12 patients (91.7%), with none requiring conversion to open surgery.
Since the first description of NOTES transanal endoscopic rectosigmoid resection in cadavers [16], extensive experimental work by our group and others demonstrated the feasibility and safety of this approach in swine [1718] and in human cadavers [19]. Transumbilical rectosigmoid resection in swine using a single port device was also developed as an intermediate technique for "pure" NOTES [20]. Single port rectosigmoidectomy in swine was safe, but remains challenging for pelvic dissection. In contrast, "pure" NOTES rectosigmoidectomy using TEM may be a promising tool for pelvic dissection [18]. Indeed, a phase 2 single-arm clinical trial showed that single-port laparoscopic sigmoidectomy through the umbilicus was safe and oncologically feasible in selected patients [21].
Our study found that the median operating time was 195 minutes, comparable to or slightly shorter than the time required for conventional laparoscopic TME [34]. Theoretically, simultaneous performance of transabdominal and transanal approaches can shorten the operation time. We found that the median time to peritoneal communication was 78 minutes, shorter than expected when testing this new technique. The operation time was reported to be shorter for transanal endoscopic TME than for conventional laparoscopic TME when the abdominal and perineal approaches were performed simultaneously [22]. This technique, however, requires the participation of 2 surgeons in a single operating room, using 2 separate laparoscopic systems for the simultaneous abdominal and perineal approaches. Moreover, this method may result in high medical costs. The estimated blood loss in our study was similar to that reported previously [358]. One patient, however, developed unexpected intraoperative bleeding while undergoing lateral dissection using the transanal approach. This bleeding was successfully controlled by gauze compression and application of a sealing device. Care must be taken to avoid dissection of the wrong plane beyond the proper lateral plane, as this can cause unexpected bleeding.
Although the morbidity rate in our patients was higher than expected, all patients were managed conservatively. Three patients developed transient ileus, with 2 managed by nasogastric tube drainage for up to 3 days and 1 without nasogastric tube drainage. Although urinary dysfunction is another possible postoperative complication, especially since the patient's anatomy may be unfamiliar when using transanal approaches, only 2 of the 12 patients (16.7%) in this study experienced urinary dysfunction, comparable to the rate after conventional laparoscopic TME (up to 20%) [23]. One patient underwent Foley catheter removal at an outpatient clinic 3 weeks after surgery, whereas another was managed medically without Foley catheter insertion.
Most early series and case reports on transanal TME found that this procedure yielded excellent resection quality, with negative margins and intact mesorectal envelopes [23]. Our preliminary series also showed excellent TME quality, comparable to that of conventional laparoscopic TME [3]. Circumferential resection margin has been associated with the outcome of TME. For example, the risk of local recurrence within 2 years after surgery was higher in patients with margins within 2 mm (16.0%) than in patients with margins greater than 2 mm (5.8%) [24]. In contrast, another study defined an involved circumferential resection margin as ≤1 mm [14]. Irrespective of the cutoff margin, only 1 of the 12 patients (8.3%) in this study had a positive circumferential resection margin, comparable to the rates reported for conventional laparoscopic surgery [34].
The key to successful dissection around the mesorectum is visualization of the loose areolar tissue surrounding the mesorectal fascia, called the "holy plane". As in the traditional top-down approach, coning in can be avoided by making this dissection at a markedly caudal angle, thus allowing for the steep angle between the anal canal and posterior levator ani muscle, requiring a steep posterior turn at the onset of the procedure [15]. In our experience, the crucial technical step is closure of the distal rectal lumen by tight purse-string sutures, preventing cancer cell contamination and air inflation of the bowel. An efficient sequence of mesorectal dissection is posterior, anterior then lateral. After partial dissection of the posterior mesorectum, the specimen can be retracted more easily, allowing clear visualization and dissection of the anterior and lateral planes. Transanal endoscopic TME is basically a type of single port surgery, which has difficulties in internal and external conflicts of instruments, tissue retraction and exposure. However, it also has the advantages of laparoscopic surgery, such as a magnified view and pneumodissection of loose connective tissues. In patients with low-lying tumors, rectal dissection was started from the intersphincteric plane without transanal platforms. In these patients, the appropriate dissection layer on the anterior side is usually obscured by intermingled muscle fibers at the level of the inferior border of the prostate and vagina, with appropriate dissection being very important to prevent urethral injury in males. After the correct dissection plane between the rectum and prostate/vagina is identified, this plane is relatively easy to maintain circumferentially, with good exposure afforded by the endoscopic view.
Surgeons performing transanal TME procedures must be trained for both laparoscopic and transanal surgeries with advanced platforms, such as TEM, transanal endoscopic operation, and transanal minimally invasive surgery [569]. The learning curve for transanal TME procedures has not been determined, although surgery on animals and human cadavers may be helpful in overcoming the learning curve. Those beginning transanal TME must learn the anatomical view during the transanal approach and be guided by more experienced surgeons in determining the correct plane. Participation in a database registry is helpful as method for personal auditing.
Only 2 of the 12 patients (16.7%) in this pilot study developed moderate to severe fecal incontinence. Both were males with high BMI and low lying tumors, requiring coloanal anastomosis. This finding is comparable with previously reported rates of fecal incontinence after transanal TME (5.7%–60.0%) [5910]. Large-scale prospective trials are needed, however, to evaluate long-term functional and oncologic outcomes.
The major limitations of this preliminary study were the small sample size and short-term follow-up. In addition, the IMA were not divided using a transanal approach, a key procedure of NOTES. This may become possible in the near future, as novel devices and instruments for transanal approaches are developed.
In conclusion, this pilot study has demonstrated the feasibility and safety of transanal endoscopic TME with laparoscopic assistance for rectal cancer. Larger multicenter prospective studies are warranted to evaluate long-term oncologic and functional outcomes.
Figures and Tables
Fig. 1
Illustration of the procedure. (A) An Octoport (Dalim, Seoul, Korea) was placed on the potential ileostomy site of the right iliac fossa. (B) A GelPOINT path (Applied Medical Inc., Rancho Santa Margarita, CA, USA) was installed at the anus after purse string suturing and full-thickness circumferential transection of the distal part of the tumor.

Fig. 2
Grading of surgical specimens. (A) The specimen removed from patient No. 1 was graded as 'complete' total mesorectal excision (TME) according to the Quirke method. (B) Due to the quality of the mesorectum, the specimen removed from patient No. 2 was graded as 'incomplete' TME.
References
1. Martling AL, Holm T, Rutqvist LE, Moran BJ, Heald RJ, Cedemark B. Effect of a surgical training programme on outcome of rectal cancer in the County of Stockholm. Stockholm Colorectal Cancer Study Group, Basingstoke Bowel Cancer Research Project. Lancet. 2000; 356:93–96.
2. Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005; 365:1718–1726.
3. van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013; 14:210–218.
4. Jeong SY, Park JW, Nam BH, Kim S, Kang SB, Lim SB, et al. Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol. 2014; 15:767–774.
5. Atallah S, Martin-Perez B, Albert M, deBeche-Adams T, Nassif G, Hunter L, et al. Transanal minimally invasive surgery for total mesorectal excision (TAMISTME): results and experience with the first 20 patients undergoing curativeintent rectal cancer surgery at a single institution. Tech Coloproctol. 2014; 18:473–480.
6. Sylla P, Rattner DW, Delgado S, Lacy AM. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc. 2010; 24:1205–1210.
7. Velthuis S, Nieuwenhuis DH, Ruijter TE, Cuesta MA, Bonjer HJ, Sietses C. Transanal versus traditional laparoscopic total mesorectal excision for rectal carcinoma. Surg Endosc. 2014; 28:3494–3499.
8. de Lacy AM, Rattner DW, Adelsdorfer C, Tasende MM, Fernández M, Delgado S, et al. Transanal natural orifice transluminal endoscopic surgery (NOTES) rectal resection: "down-to-up" total mesorectal excision (TME): short-term outcomes in the first 20 cases. Surg Endosc. 2013; 27:3165–3172.
9. Tuech JJ, K aroui M, Lelong B, De Chaisemartin C, Bridoux V, Manceau G, et al. A step toward NOTES total mesorectal excision for rectal cancer: endoscopic transanal proctectomy. Ann Surg. 2015; 261:228–233.
10. Rouanet P, Mourregot A, Azar CC, Carrere S, Gutowski M, Quenet F, et al. Transanal endoscopic proctectomy: an innovative procedure for difficult resection of rectal tumors in men with narrow pelvis. Dis Colon Rectum. 2013; 56:408–415.
11. Marks J, Nassif G, Schoonyoung H, DeNittis A, Zeger E, Mohiuddin M, et al. Sphincter-sparing surgery for adenocarcinoma of the distal 3 cm of the true rectum: results after neoadjuvant therapy and minimally invasive radical surgery or local excision. Surg Endosc. 2013; 27:4469–4477.
12. Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993; 36:77–97.
13. Quirke P, Durdey P, Dixon MF, Williams NS. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet. 1986; 2:996–999.
14. Glynne-Jones R, Mawdsley S, Novell JR. The clinical significance of the circumferential resection margin following preoperative pelvic chemo-radiotherapy in rectal cancer: why we need a common language. Colorectal Dis. 2006; 8:800–807.
15. Knol JJ, D'Hondt M, Souverijns G, Heald B, Vangertruyden G. Transanal endoscopic total mesorectal excision: technical aspects of approaching the mesorectal plane from below: a preliminary report. Tech Coloproctol. 2015; 19:221–229.
16. Whiteford MH, Denk PM, Swanström LL. Feasibility of radical sigmoid colectomy performed as natural orifice translumenal endoscopic surgery (NOTES) using transanal endoscopic microsurgery. Surg Endosc. 2007; 21:1870–1874.
17. Sylla P, Sohn DK, Cizginer S, Konuk Y, Turner BG, Gee DW, et al. Survival study of natural orifice translumenal endoscopic surgery for rectosigmoid resection using transanal endoscopic microsurgery with or without transgastric endoscopic assistance in a swine model. Surg Endosc. 2010; 24:2022–2030.
18. Sohn DK, Jeong SY, Park JW, Kim JS, Hwang JH, Kim DW, et al. Comparative study of NOTES rectosigmoidectomy in a swine model: E-NOTES vs. P-NOTES. Endoscopy. 2011; 43:526–532.
19. Telem DA, Han KS, Kim MC, Ajari I, Sohn DK, Woods K, et al. Transanal rectosigmoid resection via natural orifice translumenal endoscopic surgery (NOTES) with total mesorectal excision in a large human cadaver series. Surg Endosc. 2013; 27:74–80.
20. Leroy J, Cahill RA, Peretta S, Marescaux J. Single port sigmoidectomy in an experimental model with survival. Surg Innov. 2008; 15:260–265.
21. Park YM, Cho E, Kang HY, Kim JM. The effectiveness and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for early gastric cancer: a systematic review and metaanalysis. Surg Endosc. 2011; 25:2666–2677.
22. Fernandez-Hevia M, Delgado S, Castells A, Tasende M, Momblan D, Díaz del Gobbo G, et al. Transanal total mesorectal excision in rectal cancer: short-term outcomes in comparison with laparoscopic surgery. Ann Surg. 2015; 261:221–227.
23. Emhoff IA, Lee GC, Sylla P. Transanal colorectal resection using natural orifice translumenal endoscopic surgery (NOTES). Dig Endosc. 2014; 26:Suppl 1. 29–42.
24. Nagtegaal ID, Marijnen CA, Kranenbarg EK, van de Velde CJ, van Krieken JH, et al. Pathology Review Committee. Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit. Am J Surg Pathol. 2002; 26:350–357.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download