Abstract
Purpose
The purpose of this study was to evaluate the prognostic significance of serum CEA (s-CEA) changes in colorectal cancer (CRC) patients with sustained elevated postoperative s-CEA levels.
Methods
Between January 1999 and December 2008, 9,380 CRC patients underwent surgery. Curative resection was performed in 1,242 CRC patients with high preoperative s-CEA levels (>6 ng/mL). High s-CEA levels were normalized in 924 patients (74.4%) within 2 weeks from surgery, whereas high s-CEA levels were persistent in 318 patients (25.6%). Patients were divided into 2 groups according to their postoperative s-CEA levels: group 1 (37 patients with a 1-year postoperative s-CEA>6 ng/mL) and group 2 (281 patients with a 1-year postoperative s-CEA≤6 ng/mL).
Results
A postoperative recurrence was identified in 24 patients (64.9%) in group 1 and 65 patients (23.1%) in group 2 (P < 0.001). A curative resection after recurrence was performed in 22 patients (33.8%) from group 2, but no patients from group 1 (P = 0.001). The 5-year overall survival and time to recurrence were significantly lower in patients with recurrent cancer in group 1 (P < 0.001).
CEA is an oncofetal glycoprotein that is expressed by many epithelial tumors [1]. Since its initial description in 1965 [2], CEA has remained the most thoroughly investigated tumor marker. The protein is produced by normal fetal gut tissue and by epithelial tumors, particularly those of the large bowel. CEA is expressed in large quantities in approximately 95% of colorectal carcinomas, and the association between serum CEA (s-CEA) levels and colorectal cancer (CRC) has been studied extensively. Preoperative s-CEA levels increase with advancing tumor stage [3], and elevated preoperative s-CEA levels predict a higher incidence of recurrence in patients with CRC [4]. Furthermore, the relationship between preoperative s-CEA levels and survival suggests a predictive value of the former for metastasis or recurrence in CRC patients [56]. In some patients, high preoperative CEA levels fail to normalize following a successful surgical resection. In many instances, the precise reasons for persistent high s-CEA levels cannot be determined. The explanatory causes for this failure include overlooked metastases or an inadequate surgery [7]. Otherwise, persistent high s-CEA levels may be due to smoking, renal insufficiency, or chronic pulmonary or liver diseases [78]. The aim of our present study was to evaluate the role of preoperative and postoperative s-CEA levels as a predictor of recurrence and survival in patients with CRC.
Between January 1999 and December 2008, 9,380 CRC patients underwent surgery in Asan Medical Center, and high preoperative s-CEA levels (>6 ng/mL) were observed in 2,381 of these cases (25.4%) (Table 1). A curative resection was performed in 1,242 stages I–III patients with CRC in this population. Preoperative and postoperative s-CEA levels were retrospectively analyzed in these patients (Fig. 1). Patients who had undergone neoadjuvant chemotherapy and/or radiotherapy, patients with a known second neoplastic disease, and patients with inflammatory bowel disease were excluded. High preoperative s-CEA levels were normalized in 924 patients (74.4%) within 2 weeks from surgery. However, high s-CEA levels were present in 318 patients (25.6%) at 1- to 2-week postsurgery, and, of these, 37 patients had sustained elevated s-CEA levels until 1 year after surgery. Curative resection (R0) was defined as no gross residual tumor remaining in the surgical bed and surgical resection (both distal and circumferential) margins that were pathologically negative for tumor invasion. Patients were staged according to the criteria of the American Joint Commission on Cancer (AJCC) 7th TNM classification of malignant tumors [9].
The patients were divided into 2 groups according to their postoperative s-CEA levels. Group 1 (n = 37) was comprised of patients with a postoperative s-CEA>6 ng/mL 1 year after surgery; group 2 (n = 281) was comprised of patients with a postoperative s-CEA≤6 ng/mL 1 year after surgery. There is also no clear consensus on the frequency or duration of optimal CEA monitoring, although the current American Society of Clinical Oncology guidelines recommend monitoring every 2–3 months for at least 2 years after diagnosis. The postoperative surveillance program in our institute is as follows. Patients are followed routinely at 3- or 6-month intervals for the first 2 years and at 6- or 12-month intervals thereafter. At each visit, CEA levels are assayed, a full history is obtained, and a physical examination is performed. Colonoscopy is performed within 6 months to 1 year following surgery, and every 3 years thereafter. Abdominopelvic CT (APCT) and chest CT are performed 6 months postoperatively and then at semiannually intervals with APCT, annually intervals with chest CT. Unscheduled CT or PET scans were performed on patients with increased serum CEA concentrations or patients who were symptomatic.
Peripheral blood samples were obtained 1 week before and 2 weeks after an operation. S-CEA was measured by enzyme immunoassay (ELISA-2-CEA kit, CIS Biointernational, Marcule, France). The normal range of s-CEA in our laboratory was set as <6 ng/mL.
All data were analyzed using the IBM SPSS ver. 18.0 (IBM Co., Armonk, NY, USA). The chi-square test was used to compare clinicopathologic parameters between groups and preoperative and postoperative s-CEA levels. The cumulative survival rates were calculated by the Kaplan-Meier method. Univariate and multivariate analyses of factors associated with recurrence were performed using a multivariate logistic regression analysis and the Cox proportional hazards model to estimate hazard ratios and to yield 95% confidence intervals. Statistical significance was defined as a P-value < 0.05.
Of the 318 patients in our series with high s-CEA levels at 4 weeks postoperatively, 189 (59.4%) were males, and 129 (40.6%) were females. The mean patient age was 61.4 years (range, 28–90 years), and the median follow-up was 58 months (range, 1–174 months). Among these 320 patients also, 21 (6.5%) had stage I tumors, 133 (41.6%) had stage II tumors, and 166 (51.9%) had stage III tumors. The clinicopathologic characteristics of the patients are summarized in Table 2. Patients were further divided into 2 groups according to theirs-CEA levels 1 year postoperatively. Following curative resection, s-CEA levels were observed to normalize in 176 patients (61.9%) between 2 and 8 weeks postoperatively, in 81 patients (28.8%) between 2 and 6 months postoperatively, and in 24 patients (8.5%) between 6 and 12 months postoperatively. Postoperative management of the patients is summarized in Table 3. There was no significant difference in the chemotherapy regimens (P = 0.096) and postoperative radiation therapy (P = 0.538) between the 2 groups (P = 0.096). A significantly lower rate of chemotherapy was observed in group 1 compared with group 2. However, it did not affect the overall survival (OS) and disease free survival (P = 0.379). There was no significant difference between the comorbidity (P = 0.444) and smoking history (P = 0.650) between the groups, which were related to increased s-CEA levels.
The recurrence rate was 64.9% (24/37) and 23.1% (65/281) in groups 1 and 2, respectively (P < 0.001) and the OS rate was significantly higher (P < 0.001) in group 2 (Fig. 2). For analysis of recurrence patterns, the site of recurrence was divided into local recurrence and systemic metastasis (Table 4). There was however no significant difference between these recurrence patterns in the 2 groups (P = 0.575). Twenty-two patients in group 2 had a curative resection after recurrence compared with no patients in group 1 (P = 0.001). When metastasis were found in group 1 patients, peritoneal seeding was found in 13 patients (54%), multiple liver and lung metastasis in 9 patients (38%), and local recurrence in 2 patients (8%) with inoperable status. Multivariate logistic regression analysis showed that pathologic differentiation (P = 0.032) and abnormal postoperative s-CEA levels (P < 0.001) were independent prognostic factors for a postoperative recurrence (Table 5). Patients in group 1 had a 7.2 fold higher risk of recurrence than patients in group 2. The patients with relapse within 1 year were 20/37 in group 1 and 25/281 in group 2. Being classified in group 1 was identified as an independent prognostic factor for survival in a Cox proportional hazards regression model (Table 5). The patients with death within 1 year were 7 of 37 in group 1 and 7 of 281 in group 2. The 924 patients with normalized postoperative s-CEA levels clearly demonstrated a lower recurrence rate (122 patients, 13.2%). Among the 122 patients with recurrence, liver and lung metastasis were found in 89 patients (73.0%), peritoneal seeding in 19 patients (15.6%), and local recurrence in 14 patients (11.4%).
We found in our present study that persistently elevated postoperative s-CEA levels were significantly correlated with high recurrence and low survival rates in CRC patients. In 25.6% (318 of 1,242) of stages I–III CRC patients with abnormal preoperative s-CEA levels, elevated s-CEA levels were sustained postoperatively and patients with high pre- and postoperative s-CEA levels exhibited a poorer outcome. Moreover, multivariate analysis revealed that abnormal postoperative s-CEA levels and tumor differentiation were predictive factors for recurrence. Furthermore, a persistently high postoperative s-CEA level was the only predictive factor for survival. An initially elevated s-CEA level that fails to normalize in the early postoperative period following a curative resection has been suggested to represent an additional relevant risk factor [10]. Several studies report that elevated preoperative and postoperative s-CEA levels are associated with more advanced disease and with an adverse outcome following a successful surgical resection [1112].
In 1999, the College of American Pathologists consensus statement reported preoperative s-CEA elevation as a category I prognostic factor, with category I factors including "definitively proven to be of prognostic import based on evidence from multiple statistically robust published trials and generally used in patient management [13]." However, preoperative s-CEA was the only category I factor not included in the current AJCC staging system [14]. According to the European Group on Tumor Markers guidelines, s-CEA is considered to be of independent prognostic value [15]. Other reports have suggested that advanced stage tumors significantly correlated with higher preoperative and postoperative s-CEA levels. In our present study, the proportion of patients with an elevated preoperative s-CEA level correlated with stage advancement (3.7% in stage I vs. 47.8% in stage IV). The detection rate of abnormal preoperative s-CEA levels appeared to increase with tumor stage progression. We and others have suggested that s-CEA levels should be added to the current staging system [161718].
Surveillance programs following primary resection are important in the early identification of recurrent disease and distant metastasis while the tumors are still asymptomatic and potentially curable. Despite a variety of strategies and proposed schedules, objective data for surveillance programs remain limited. Early detection of CRC relapse is an important factor in reducing cancer mortality [7]. Intensive follow-up after a curative resection of CRC improves OS and the re-resection rate for recurrent disease [19]. The level of postoperative CEA has been shown to correlate with metastasis and local recurrence [2021]. S-CEA has shown promise as an indicator of residual disease before recurrence becomes clinically apparent, and patients with recurrence might have a greater chance of being cured if the residual disease is identified and treated effectively at an earlier time. A rise in postoperative CEA levels prior to clinically observable recurrence have been reported in 18%–75% of cases with CRC relapse [2022]. This rise has been reported as early as 2.5–4 months prior to recurrence and may indicate the potential of a recurrence, possibly detecting a relapse at an early stage [2123]. Frequent monitoring of CEA postoperatively may allow for the identification of patients with metastatic disease for whom surgical resection or other localized therapy may be potentially beneficial. Our current findings demonstrated that patients with persistently elevated s-CEA levels have a significantly high recurrence rate. Extended surveillance with sensitive diagnostic techniques and treatment of recurrences at an earlier stage of disease should be performed in these patients.
In our present analyses also, patients with persistently elevated s-CEA levels showed a significantly high recurrence rate after surgery. Furthermore, a curative resection after a recurrence was not achieved in any of these patients. The OS rate of patients with persistently high s-CEA levels after a recurrence was significantly different from that of patients with persistently low s-CEA levels. Other factors may affect postoperative s-CEA concentrations in CRC patients. S-CEA may be increased in epithelial tumors at other sites (both benign and malignant), inflammatory bowel disease, pancreatitis, liver disease, pulmonary infection, bowel obstruction, and in smokers [24]. Patients with postoperative complications, such as pulmonary disorders (e.g., pneumonia, pleural effusion, and atelectasis), toxic hepatitis and abnormal renal functions due to anesthesia, and mechanical or paralytic bowel obstruction caused by surgery, also display increased s-CEA levels. In our current study, patients with a known epithelial tumor and with inflammatory bowel disease were excluded from the analysis. There was no statistically significant difference between comorbidities, including liver disease, chronic obstructive lung disease, and smoking history. However, there were patients with postoperative complications, such as pulmonary disorders (including pneumonia, pleural effusion, and atelectasis), hepatitis or abnormal renal functions due to anesthesia, and mechanical or paralytic bowel obstruction caused by surgery. These causes may be related to increased postoperative s-CEA levels, but did not demonstrate local recurrence or metastasis in our patients.
We found in our present series that 24 patients in group 1 had a recurrence, and 13 patients in this group showed no evidence of recurrence. Among these 13 patients, only 4 patients (4 of 37, 11%) showed no evidence of recurrence from physical results, laboratory findings, and imaging studies after 5 years and still survive. These patients were not smokers and had no other comorbidities such as liver disease, lung problems, or other malignancies. Among the remaining 9 patients in group 1 who did not have recurrence, 1 patient was lost to follow-up and 8 patients were died from unknown causes.
This study had several limitations of note. First, our analysis was retrospective in nature. Second, adjuvant chemoradiation therapy differs between oncologists, and different regimens were used in our patient cohort.
In conclusion, persistently high postoperative s-CEA levels are a clinically important predictor of poor prognosis in CRC patients. Extensive surveillance of these patients with a high postoperative s-CEA of unknown cause is recommended, and consider aggressive adjuvant chemotherapy without confirming if recurrence is suspected. However, long-term follow-up studies in a large CRC cohort are additionally required to strengthen this hypothesis.
Figures and Tables
 | Fig. 1Study algorithm used to select colorectal cancer patients with an elevated preoperative serum CEA level. |
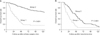 | Fig. 2Cumulative overall survival rates in colorectal cancer patients with an elevated preoperative serum CEA (group1, still elevated at 1-year postsurgery; group 2, normalized by 1-year postsurgery), after primary surgery (A) and recurrence (B), respectively. |
Table 2
Clinicopathologic characteristics of the study patients

Values are presented as number (%) or mean ± standard deviation. Group 1, persistent elevated s-CEA at 1-year postsurgery; group 2, normalized s-CEA at 1-year postsurgery; s-CEA, serum CEA; LN, lymph node; WD, well differentiated; MD, moderately differentiated; PD, poorly differentiated; SRC, signet ring cell; Muc, mucinous; COPD, chronic obstructive lung disease.
References
1. Chen CH, Hsieh MC, Lai CC, Yeh CY, Chen JS, Hsieh PS, et al. Lead time of carcinoembryonic antigen elevation in the postoperative follow-up of colorectal cancer did not affect the survival rate after recurrence. Int J Colorectal Dis. 2010; 25:567–571.
2. Gold P, Freedman SO. Specific carcinoembryonic antigens of the human digestive system. J Exp Med. 1965; 122:467–481.
3. Palmqvist R, Engaras B, Lindmark G, Hallmans G, Tavelin B, Nilsson O, et al. Prediagnostic levels of carcinoembryonic antigen and CA 242 in colorectal cancer: a matched case-control study. Dis Colon Rectum. 2003; 46:1538–1544.
4. Wanebo HJ, Rao B, Pinsky CM, Hoffman RG, Stearns M, Schwartz MK, et al. Preoperative carcinoembryonic antigen level as a prognostic indicator in colorectal cancer. N Engl J Med. 1978; 299:448–451.
5. Moertel CG, O'Fallon JR, Go VL, O'Connell MJ, Thynne GS. The preoperative carcinoembryonic antigen test in the diagnosis, staging, and prognosis of colorectal cancer. Cancer. 1986; 58:603–610.
6. Carriquiry LA, Piñeyro A. Should carcinoembryonic antigen be used in the management of patients with colorectal cancer? Dis Colon Rectum. 1999; 42:921–929.
7. Wang JY, Lin SR, Wu DC, Lu CY, Yu FJ, Hsieh JS, et al. Multiple molecular markers as predictors of colorectal cancer in patients with normal perioperative serum carcinoembryonic antigen levels. Clin Cancer Res. 2007; 13:2406–2413.
8. Wang WS, Lin JK, Chiou TJ, Liu JH, Fan FS, Yen CC, et al. Preoperative carcinoembryonic antigen level as an independent prognostic factor in colorectal cancer: Taiwan experience. Jpn J Clin Oncol. 2000; 30:12–16.
9. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17:1471–1474.
10. Slentz K, Senagore A, Hibbert J, Mazier WP, Talbott TM. Can preoperative and postoperative CEA predict survival after colon cancer resection? Am Surg. 1994; 60:528–531.
11. Wang JY, Lu CY, Chu KS, Ma CJ, Wu DC, Tsai HL, et al. Prognostic significance of pre- and postoperative serum carcinoembryonic antigen levels in patients with colorectal cancer. Eur Surg Res. 2007; 39:245–250.
12. Filiz AI, Sucullu I, Kurt Y, Karakas DO, Gulec B, Akin ML. Persistent high postoperative carcinoembryonic antigen in colorectal cancer patients: is it important? Clinics (Sao Paulo). 2009; 64:287–294.
13. Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000; 124:979–994.
14. Chen CC, Yang SH, Lin JK, Lin TC, Chen WS, Jiang JK, et al. Is it reasonable to add preoperative serum level of CEA and CA19-9 to staging for colorectal cancer? J Surg Res. 2005; 124:169–174.
15. Duffy MJ, van Dalen A, Haglund C, Hansson L, Klapdor R, Lamerz R, et al. Clinical utility of biochemical markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines. Eur J Cancer. 2003; 39:718–727.
16. Wiratkapun S, Kraemer M, Seow-Choen F, Ho YH, Eu KW. High preoperative serum carcinoembryonic antigen predicts metastatic recurrence in potentially curative colonic cancer: results of a five-year study. Dis Colon Rectum. 2001; 44:231–235.
17. Watine J, Miedouge M, Friedberg B. Carcinoembryonic antigen as an independent prognostic factor of recurrence and survival in patients resected for colorectal liver metastases: a systematic review. Dis Colon Rectum. 2001; 44:1791–1799.
18. Louhimo J, Carpelan-Holmstrom M, Alfthan H, Stenman UH, Jarvinen HJ, Haglund C. Serum HCG beta, CA 72-4 and CEA are independent prognostic factors in colorectal cancer. Int J Cancer. 2002; 101:545–548.
19. Tjandra JJ, Chan MK. Follow-up after curative resection of colorectal cancer: a meta-analysis. Dis Colon Rectum. 2007; 50:1783–1799.
20. Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Tangen C. An evaluation of the carcinoembryonic antigen (CEA) test for monitoring patients with resected colon cancer. JAMA. 1993; 270:943–947.
21. McCall JL, Black RB, Rich CA, Harvey JR, Baker RA, Watts JM, et al. The value of serum carcinoembryonic antigen in predicting recurrent disease following curative resection of colorectal cancer. Dis Colon Rectum. 1994; 37:875–881.
22. Wedell J, Meier zu Esssen P, Luu TH, Fiedler R, van Calker H, Koldowski P, et al. A retrospective study of serial CEA determinations in the early detection of recurrent colorectal cancer. Dis Colon Rectum. 1981; 24:618–621.
23. Park IJ, Choi GS, Lim KH, Kang BM, Jun SH. Serum carcinoembryonic antigen monitoring after curative resection for colorectal cancer: clinical significance of the preoperative level. Ann Surg Oncol. 2009; 16:3087–3093.
24. Rockall TA, McDonald PJ. Carcinoembryonic antigen: its value in the follow-up of patients with colorectal cancer. Int J Colorectal Dis. 1999; 14:73–77.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download