Abstract
Purpose
The purpose of this study was to examine 2-year follow-up results of cytoreductive surgery (CRS) and intraperitoneal chemotherapy (IPC) for peritoneal carcinomatosis (PC) of colorectal cancer.
Methods
We performed 54 cases of CRS and IPC in 53 patients with PC of colorectal cancer from December 2011 to December 2013. We collected data prospectively and analyzed the grade of PC, morbidity and mortality, and short-term follow-up (median, 10 months; range, 2–47 months) results.
Results
Mean peritoneal cancer index (PCI) was 15 (range, 1–35), and complete cytoreduction was possible in 35 patients (64.8%). Complications occurred in 25 patients (46.3%) and mortality occurred in 4 patients (7.4%). Excluding the 4 mortalities, 17 patients out of 49 patients (31.5%) were alive at the time of the last follow-up and the overall median survival was 10.3 months. Patients with complete cytoreduction had a median survival of 22.6 months, which was significantly longer than the median survival of 3.5 months for patients without complete cytoreduction (P < 0.001). PCI grade, CCR grade, cell type, and postoperative chemotherapy were significant prognostic factors by univariate analysis. Positive independent prognostic factors by multivariate analysis included PCI grade and postoperative chemotherapy.
Conclusion
CRS and IPC increased the survival of patients with low PCI and postoperative systemic chemotherapy was mandatory. However, this combined therapeutic approach showed high rate of complications and mortality. Therefore, this aggressive treatment should be performed in only selected patients by considering the general condition of the patient and the extent of PC.
Peritoneal carcinomatosis (PC) is one of the most common forms of colon cancer metastasis, and about 10%–35% of PC is found in a solitary form without other metastasis. Its prognosis is extremely poor with a mean life expectancy of only 6 months. Up until now, standard treatment for PC is limited to systemic chemotherapy with minimal efficacy even in the era of modern systemic chemotherapy. Though PC has been considered as a systemic disease, Sugarbaker proposed that PC is still locoregional disease and more aggressive locoregional treatment is needed. Current multimodality therapy combines cytoreductive surgery (CRS) to remove all visible tumors, with intraperitoneal chemotherapy (IPC) to eradicate microscopic residual disease. Such multimodality therapy has demonstrated the best results for PC through many studies, and some countries have adopted the combination of CRS and hyperthermic intraperitoneal chemotherapy (HIPEC) in official treatment guidelines for colorectal cancer patients with PC. Nevertheless, it has not been universally embraced by the medical community, and most hospitals in Korea do not practice CRS and IPC [1234]. We started CRS and IPC from early 2000, and the case number was increased from end of 2011. The purpose of this study was to obtain short-term follow-up results of CRS and IPC for PC of colorectal cancer.
We performed CRS and IPC for 68 patients between December 2011 and December 2013. Among them, 53 patients (77.9%) with PC of colorectal origin had undergone 54 procedures. One patient underwent second CRS and IPC for recurrence of PC. The inclusion criteria were histologically confirmed PC of colorectal origin treated with CRS plus early postoperative intraperitoneal chemotherapy (EPIC) or HIPEC. Exclusion criteria were as follow: presence of extra-abdominal metastasis, presence of unresectable hepatic metastasis, and poor general condition (Eastern Cooperative Oncology Group performance status >2).
We collected data prospectively and analyzed the grade of PC, morbidity and mortality, and short-term follow-up (median, 10 months; range, 2–47 months) results. The extent of PC was assessed through intraoperative exploration by using peritoneal cancer index (PCI) with a score of 0 to 3 for each of the 13 defined areas of the abdominal cavity. Therefore, the total PCI ranged from 0 to 39 [5]. Three PCI grades (0–10, 11–20, and >20) were used for analysis. The completeness of cytoreduction (CCR) was assessed using the CCR Score. Index that quantifies the extent of residual disease at the end of the procedure is classified into 4 categories: CCR0 (no macroscopic residual disease); CCR1 (residual tumors less than 2.5 mm); CCR2 (residual tumors between 2.5 mm and 2.5 cm); CCR3 (residual tumors greater than 2.5 cm) [5]. CCR0 and CCR1 represent complete cyoreduction while CCR2 and CCR3 are designed as incomplete cytoreduction. Morbidity was assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events v4.0. Possible reasons of mortality were described in details below.
Preoperative work-up including chest CT, abdominopelvic CT and PET-CT were performed to evaluate peritoneal disease and distant metastasis. Patients also underwent detailed preoperative evaluation to assess physical condition and performance status. Contraindications to the procedure included the presence of unresectable extra-abdominal metastasis, unresectable intra-abdominal metastasis, or poor general condition (Eastern Cooperative Oncology Group performance status >2). All patients gave their informed consent about the risk and benefit of the procedure, available other treatments, and reimbursement.
CRS consisted of the major peritonectomy procedures proposed by Sugarbaker (Fig. 1) [6]. EPIC was delivered for 5 days (from day 1 to day 5 after surgery). The chemotherapeutic agent used for EPIC was mitomycin on day 1 and 5-fluorouracil for the following 4 days. The HIPEC was done by closed method using Belmont Hyperthermic Pump (Belmont Instrument Corop., Billerica, MA, USA) at the end of the surgery under general anesthesia (Fig. 2). For HIPEC, mitomycin was used. The duration of perfusion was 90 minutes. The inflow temperature was 42℃–43℃. All patients were admitted to the intensive care unit for at least 24 hours.
Categorical variables were described in terms of frequency and percentage. The distributions of continuous variables were described with mean, standard error, and median. Survival analysis was performed using the Kaplan-Meier method, and comparison of curve was made with log-rank test.
Cox proportional hazard regression model was used for multivariate analysis of prognostic factors. A P-value of less than 0.05 was considered as statistically significant. Statistical analyses were performed using the IBM SPSS Statistics ver. 23.0 (IBM Co., Armonk, NY, USA).
Patient characteristics are summarized in Table 1. The median age of patients was 56 (range, 36–79 years) years old and 25 (46.3%) were females. PC was synchronously detected in 29 patients (53.7%). A total of 31 patients (57.4%) were previously treated for PC. Mucinous, signet ring cell, or poorly differentiated PC cell types were found in 23 patients (42.6%). Mean PCI was 15 (range, 1–35).
At the completion of CRS, complete cytoreduction was achieved in 35 patients (64.8%). Forty-seven patients underwent EPIC, 3 patients underwent HIPEC, and 5 patients underwent both. The mean operation time was 7 hours. Forty-two patients (77.8%) received postoperative systemic chemotherapy: FOLFOX ± target agents in 15 patients, FOLFIRI ± target agents in 12 patients, others in 15 patients (5-FU, leucovorin [FL], Capecitabine, target agent only, etc.). Details of treatment are shown in Table 1.
Complications occurred in 25 patients (46.3%) and 12 (22.2%) were complications of above grade 3. Pulmonary complications such as pleural effusion and pneumonia were the most common (29.6%) complications, followed by acute renal failure (14.8%), wound problem (13.0%), and prolonged ileus (11.1%). Anastomotic leakage was developed in 1 patient. Mortality occurred in 4 patients (7.4%). The cause of death included pulmonary complication (n = 2), septic shock related to anastomotic leakage (n = 1), and hypovolemic shock from gastrointestinal bleeding (n = 1).
Excluding the 4 mortalities, 17 (31.5%) out of the remaining 49 patients were alive at the time of the last follow-up with an overall median survival time of 10.3 months. The overall 2-year and 3-year survival rates were 31% and 28%, respectively (Fig. 3). Patients with complete cytoreduction had a median survival of 22.6 months, which was significantly longer than the median survival of 3.5 months for patients without complete cytoreduction (P < 0.001, Fig. 4). Location of primary tumor, PCI grade, CCR grade, cell type, and adjuvant chemotherapy were significant prognostic factors by univariate analysis (Table 2). Positive independent prognostic factors by multivariate analysis included PCI grade and adjuvant chemotherapy (Table 3).
Synchronous PC is found in 10%–15% of colorectal cancer patients. Metachronous peritoneal metastasis is detected in 50% of patients with recurrent colorectal cancer after curative resection. About 10%–35% of recurrence is found in a form of solitary peritoneal metastasis without any evidence of metastasis to other organs [1234]. Even though peritoneal metastasis is one of the most common forms of colon cancer metastasis, its prognosis is extremely poor with a mean life expectancy of only 6 months. Up until now, mainstream treatment standard for patients with PC is limited to systemic chemotherapy that shows minimal efficacy due to poor vascular anatomical character of peritoneum [1234].
Hence, attempts have been made to maximize the antitumor effect by radically resecting grossly visible tumors with CRS and subsequently applying highly concentrated chemo-agents directly into the peritoneal cavity [6]. It has been reported that elevating the temperature of abdominal cavity during IPC can enhance the antitumor effect of chemo-agents. Recently, it has been reported that HIPEC can extend the median survival time up to 30 months and achieve 20%–30% of 5-year survival rate [7]. Thus, CRS accompanied with IPC might be the most effective treatment proven for PC to date. Based on these results, some countries have adopted the combination of CRS and HIPEC in official treatment guidelines for colorectal cancer patients with PC [89].
However, most hospitals in Korea or other countries do not practice this treatment modality. A few disputes need to be settled in this regard. First, this multimodality therapy has insufficient evidence to be properly compared to systemic chemotherapy. Most studies up until now are retrospective observational studies [101112]. To the best of our knowledge, the studies of Verwaal et al. [1314] are the only ones that have been conducted with randomized control method. Furthermore, systemic chemotherapy in the studies of Verwaal et al. [1314] was based solely on FL regimen, hence reflecting the absolute necessity of randomized controlled studies on oxaliplatin and irinotecan that are currently widely used in metastatic colon cancer. In addition, this treatment is not an alternative to systemic chemotherapy. Rather, it is a combination of systemic chemotherapy in addition to multimodality therapy to achieve a better outcome of the treatment [1516]. The second issue is the risk of the multimodality therapy itself. Its complication rate and morbidity rate are indeed relatively high. Recent reports, however, indicate that there is no significant difference in its risks compared to other major operations such as major hepatic resection [1718]. Considering the fact that leaving peritoneal metastasis untreated is also another risk for the patient, risk of multimodality therapy should not be used as a reason not to practice it. Third, the methods and procedures used for CRS and IPC are different from hospital to hospital. To solve this problem, we need to standardize and verify the treatment algorithm. Such verification process is currently in progress in various countries [919]. There are 2 types of IPC method: EPIC and HIPEC. EPIC has the advantage of being easy to perform. The disadvantage of HIPEC is that special and costly equipment is required for hyperthermia and the duration of operation time is longer. Although no randomized study has compared these 2 procedures, Elias et al. [20] suggest that HIPEC is less dangerous and more efficient. The fourth is the selection of patients. As mentioned earlier, multimodality therapy entails high risks. Therefore, it needs to be applied to selected patients who are expected to have satisfactory results. The most important prognostic factor for predicting the result of the treatment at present is the CCR because it is related to the severity of PC [12]. Therefore, in patients whose preoperative evaluation and intraoperative findings indicate that complete cytoreduction is unattainable, this treatment should not be considered. The peritoneal metastasis that is judged to be completely resectable, unresectable other organ metastasis, and satisfactory general performance would be the optimum candidate eligible for this therapy. The final issue of concern is preoperative evaluation of peritoneal metastasis. Diagnostic tools that are currently in use such as CT or PET-CT have low accuracies with regards to assessment for the severity of PC. They have the tendency to underestimate the extent [2122]. Accordingly, future research is required to accurately evaluate the extent of PC by means of radiologic imaging and tumor marker study.
Although it is true that there are some disputes over multimodality therapy, considering the fact that its efficacy has been confirmed, it is about time for use to standardize the treatment of PC by encompassing systemic chemotherapy and multimodality therapy. Discussion through multidisciplinary committee is mandatory in deciding the method and sequences of treatment. In addition, patients should be fully informed about the risk and benefit of this treatment. They should at least be given a chance to choose their treatment option. Since it has been reported that the survival and complication rates are related to the skill of medical staff members, patients should be promptly referred to specialized hospitals for multidisciplinary treatment once peritoneal metastasis is diagnosed [23].
Our study inevitably carries some problems and limitations. First, the outcome of our study showed relatively short median survival time and rather high morbidity and mortality rates due to the fact that many of our patients already received longterm systemic chemotherapy prior to surgeries. In addition, the extent of peritoneal metastasis in many of our patients was severe. As the experience of medical staff members is known to be strongly relevant with the outcome, complication rate and mortality rate are steadily decreasing in our clinic since 2013. Second, the CCR known to be the most important prognostic factor was significantly related to survival time in our univariate analysis. However, it was not proven to be an independent prognostic factor in multivariate analysis. The reason is currently unclear. Standardization and verification are needed to decide the CCR in the operation field. In addition, 3 patients with appendiceal cancer were included in our study, suggesting problem in the selection of patients. A change was made in the method of IPC in our clinic with the adoption of HIPEC in 2013. It was applied in eight patients of this study. Finally, our data were collected prospectively. However, this study was an observation study without a control group.
Although some disputes and limitations remain for multimodality therapy, this study has its significance as the first attempt in Korea to report the result of multimodality therapy for colorectal cancer patients with peritoneal metastasis. CRS and IPC increased the survival time of patients with low PCI and postoperative systemic chemotherapy was mandatory. However, this combined therapeutic approach showed high rate of complications and mortalities. Therefore, we believe that this aggressive treatment should be performed in selected patients only by considering their general condition and the extent of PC. Our future aim is to execute thorough inspection for patients' selection and make efforts to enhance surgical technique through peritonectomy surgical symposium. We also propose a multicenter study to evaluate the effect of multimodality therapy.
Figures and Tables
Fig. 1
Operative findings of cytoreductive surgery for peritoneal carcinomatosis from sigmoid colon cancer: (A) right upper quadrant peritonectomy with cholecystectomy, (B) left upper quadrant peritonectomy with splenectomy, (C) pelvic peritonectomy with low anterior resection, total abdominal hysterectomy, and bilateral salpingoophorectomy, and (D) operation specimen.
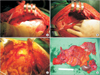
Fig. 2
Hperthermic intraperitoneal chemotherapy using the Belmont Hyperthermic Pump (Belmont Instrument Corp., Billerica, MA, USA).

Fig. 3
Overall survival for 49 patients after cytoreductive surgery and intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal cancer.

Fig. 4
Overall survival according to completeness of cytoreduction. Complete cytoreduction group (CCR 0–1) had a better survival than incomplete cytoreduction group (CCR 2–3) (median survival: 22.6 months vs. 3.5 months, P < 0.001).

Table 2
Factors associated with overall survival after cytoreductive surgery and intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal cancer: univariate analysis

References
1. Lungoci C, Mironiuc AI, Muntean V, Oniu T, Leebmann H, Mayr M, et al. Multimodality treatment strategies have changed prognosis of peritoneal metastases. World J Gastrointest Oncol. 2016; 8:67–82.
2. Mirnezami R, Mehta AM, Chandrakumaran K, Cecil T, Moran BJ, Carr N, et al. Cytoreductive surgery in combination with hyperthermic intraperitoneal chemotherapy improves survival in patients with colorectal peritoneal metastases compared with systemic chemotherapy alone. Br J Cancer. 2014; 111:1500–1508.
3. Weber T, Roitman M, Link KH. Current status of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in patients with peritoneal carcinomatosis from colorectal cancer. Clin Colorectal Cancer. 2012; 11:167–176.
4. Roviello F, Caruso S, Marrelli D, Pedrazzani C, Neri A, De Stefano A, et al. Treatment of peritoneal carcinomatosis with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: state of the art and future developments. Surg Oncol. 2011; 20:e38–e54.
5. Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996; 82:359–374.
6. Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995; 221:29–42.
7. Cao C, Yan TD, Black D, Morris DL. A systematic review and meta-analysis of cytoreductive surgery with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol. 2009; 16:2152–2165.
8. Esquivel J, Piso P, Verwaal V, Bachleitner-Hofmann T, Glehen O, González-Moreno S, et al. American Society of Peritoneal Surface Malignancies opinion statement on defining expectations from cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with colorectal cancer. J Surg Oncol. 2014; 110:777–778.
9. Esquivel J, Sticca R, Sugarbaker P, Levine E, Yan TD, Alexander R, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal surface malignancies of colonic origin: a consensus statement. Society of Surgical Oncology. Ann Surg Oncol. 2007; 14:128–133.
10. Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA, De Simone M, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004; 22:3284–3292.
11. Elias D, Gilly F, Boutitie F, Quenet F, Bereder JM, Mansvelt B, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010; 28:63–68.
12. Cavaliere F, De Simone M, Virzì S, Deraco M, Rossi CR, Garofalo A, et al. Prognostic factors and oncologic outcome in 146 patients with colorectal peritoneal carcinomatosis treated with cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy: Italian multicenter study S.I.T.I.L.O. Eur J Surg Oncol. 2011; 37:148–154.
13. Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003; 21:3737–3743.
14. Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008; 15:2426–2432.
15. Franko J, Ibrahim Z, Gusani NJ, Holtzman MP, Bartlett DL, Zeh HJ 3rd. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer. 2010; 116:3756–3762.
16. Chua TC, Morris DL, Saxena A, Esquivel J, Liauw W, Doerfer J, et al. Influence of modern systemic therapies as adjunct to cytoreduction and perioperative intraperitoneal chemotherapy for patients with colorectal peritoneal carcinomatosis: a multicenter study. Ann Surg Oncol. 2011; 18:1560–1567.
17. Chua TC, Yan TD, Saxena A, Morris DL. Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure?: a systematic review of morbidity and mortality. Ann Surg. 2009; 249:900–907.
18. Canda AE, Sokmen S, Terzi C, Arslan C, Oztop I, Karabulut B, et al. Complications and toxicities after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2013; 20:1082–1087.
19. Turaga K, Levine E, Barone R, Sticca R, Petrelli N, Lambert L, et al. Consensus guidelines from The American Society of Peritoneal Surface Malignancies on standardizing the delivery of hyperthermic intraperitoneal chemotherapy (HIPEC) in colorectal cancer patients in the United States. Ann Surg Oncol. 2014; 21:1501–1505.
20. Elias D, Benizri E, Di Pietrantonio D, Menegon P, Malka D, Raynard B. Comparison of two kinds of intraperitoneal chemotherapy following complete cytoreductive surgery of colorectal peritoneal carcinomatosis. Ann Surg Oncol. 2007; 14:509–514.
21. Pfannenberg C, Königsrainer I, Aschoff P, Oksüz MO, Zieker D, Beckert S, et al. (18)F-FDG-PET/CT to select patients with peritoneal carcinomatosis for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2009; 16:1295–1303.
22. Lim JS, Kim MJ, Yun MJ, Oh YT, Kim JH, Hwang HS, et al. Comparison of CT and 18F-FDG pet for detecting peritoneal metastasis on the preoperative evaluation for gastric carcinoma. Korean J Radiol. 2006; 7:249–256.
23. Kusamura S, Baratti D, Hutanu I, Rossi P, Deraco M. The importance of the learning curve and surveillance of surgical performance in peritoneal surface malignancy programs. Surg Oncol Clin N Am. 2012; 21:559–576.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download