Abstract
We describe 2 cases of patients with loss of hepatic arterial flow during surgery for pancreatic head cancer due to celiac stenosis caused by median arcuate ligament compression. The first case underwent pylorus-resecting pancreatoduodenectomy for pancreatic head cancer. After resection of the gastroduodenal artery, flow in the common hepatic artery disappeared, and celiac axis stenosis was identified. Interventional stent insertion was attempted, however, it failed due to the acute angle of the celiac orifice (os). This problem was resolved by arterial reconstruction. The second case underwent pylorus-preserving pancreatoduodenectomy for pancreatic head cancer and the same phenomenon occurred during the procedure. Interventional stent insertion was also tried; in this patient, however, it failed due to the acute angle of the celiac os. The problem was resolved by changing a femoral approach to a brachial approach, and the stent was inserted into the celiac os successfully.
Celiac artery occlusion or stenosis is identified in approximately 12.5%–49% (2%–24%) of all individuals undergoing abdominal angiography [12]. If there is no vascular anatomic variation, the celiac artery supplies blood to the upper abdominal organs such as the liver, stomach, duodenum and spleen. However, in general, the celiac artery has no clinical significance, owing to collateral pathways such as pancreaticoduodenal arcades or dorsal pancreatic artery and embryological communication between the celiac axis and the superior mesenteric artery (SMA) [3]. There are various causes of celiac stenosis, and they can be either vascular or nonvascular. The most common causes of celiac axis stenosis (CAS) are arteriosclerosis in Western countries and median arcuate ligament (MAL) compression in Eastern countries [45]. Among the various causes of CAS, MAL compression is found in up to 5% of patients during pancreatoduodenectomy (PD) [678]. The MAL is a band of connective tissue that runs transversely across the aorta and connects the diaphragmatic crura [7]. MAL compression was first described in 1963. This phenomenon is due to the celiac axis arising from the aorta at a higher level than normal, or it can occur if the MAL is located at a lower position than normal [9]. A few published reports have described CAS or occlusion due to MAL compression and its treatment. In this report, we describe two cases of patients with CAS due to MAL compression during surgery for pancreatic cancer at the pancreatic head.
A 57-year-old woman was referred to our institution with a 2-month history of painless jaundice. Other than that, she was healthy. Her serum total bilirubin level was 6.5 mg/dL, her CA 19-9 concentration was 3,243 U/mL, and her CEA level was 6.9 ng/mL. Preoperative abdominal CT revealed a mass in the pancreatic head with the double duct sign. The patient underwent a pylorus-resecting PD (PrPD). Intraoperatively, after the gastroduodenal artery (GDA) was resected, a significant decrease in blood flow through the hepatic artery proper was detected. MAL was incidentally observed to be compressing the celiac axis. Therefore, we released the MAL and subsequently finished the PD. However, the celiac trunk was found to be hypotrophic and failed to fully expand, and the patient's arterial flow did not recover. The patient was transferred to the angio-intervention room while under general anesthesia so that an angiographic intervention for her celiac stenosis could be performed. Angiography showed severe stenosis of the celiac orifice (os). Stent insertion failed, however, because of the acute angle of the celiac os. We decided to take the patient back to the operating room and inserted the celiac stent successfully through the GDA stump intraoperatively (Fig. 1). The return of flow in the hepatic artery proper was checked and we found that the flow in the common hepatic artery (CHA) just after the procedure had disappeared. For occlusion of CHA, because approaching the celiac trunk was impossible due to the stent, we performed a segmental resection and created an anastomosis between the CHA and aorta. The patient's arterial flow recovered. She was discharged without complications on the day 18 postoperatively. Her most recent follow-up CT performed about 2 years postoperatively showed that her arterial flow was patent (Fig. 2).
A 56-year-old man visited our institution for the evaluation and treatment of indigestion that had started a month earlier. His serum total bilirubin level was 1.0 mg/dL, his CA 19-9 concentration was 284 U/mL, and his CEA level was 3.5 ng/mL. Preoperative abdominal CT revealed an ill-defined, low attenuation lesion at the head of the pancreas with distal pancreatic duct dilation that suggested pancreatic cancer. The patient had an abnormality in his vascular anatomy; the celiac os was not shown clearly, and the GDA originated from the SMA. He underwent pylorus-preserving PD (PPPD). During the operation, flow in the hepatic artery proper was not detected after resection of the GDA. Angiography was performed just after the operation while the patient was under general anesthesia. Severe stenosis of the celiac os was found. A femoral artery approach for ballooning and stent insertion failed because of the acute angle and stenosis of the celiac os. Therefore, the vascular approach was changed from the femoral to the left brachial approach, and stent insertion into the celiac trunk was performed successfully (Fig. 3). The patient was discharged without complications on the 28th day postoperatively. His last CT performed 14 months postoperatively showed that his arterial flow was patent; however, he died 15 months postoperatively due to the recurrence of the tumor.
Celiac artery occlusion or stenosis is identified in approximately 12.5%–49% of all individuals who undergo abdominal angiography [12]. In the case of CAS, antegrade blood flow is reduced through the CHA and GDA. However, this has no clinical significance, owing to collateral pathways that develop from the SMA via the inferior pancreaticoduodenal artery to provide retrograde flow through the GDA to the liver [6]. CAS is diagnosed by measuring the diameter of the stenotic and normal parts of the celiac axis, length of the stenotic part, and distance from the aorta.
Sugae et al. [6] presented the classification of MAL compression based on a 3-dimensional CT angiography. This classification scheme has 3 types. The factors considered are rate of stenosis, length of stenosis, distance from the aorta, whether collateral pathways exist, and expected procedures. The treatment method differs according to the type of stenosis. There are various causes of celiac stenosis: (1) extrinsic stenosis due to compression by MAL or the celiac ganglion, (2) intrinsic stenosis due to atherosclerosis, and (3) other causes, including congenital causes, tumor invasion, compression by a swollen pancreas in patients with pancreatitis, or injury during angiography [45]. The most common cause of CAS in Western countries is arteriosclerosis [5]. Among the various causes, celiac artery stenosis due to MAL compression is rare. A few cases of celiac axis occlusion due to MAL compression and its treatment have been reported. Park et al. [4] described the etiology of CAS in Korean patients with liver tumors. They reported the causes to be extrinsic compression in 55% of patients, intrinsic stenosis in 10%, and other causes in 35%.
For CAS or occlusion due to MAL compression, MAL division during surgery can be considered the primary procedure. Because this phenomenon appears as the result of an anatomic abnormality between the aorta and ligaments arising from the aorta, the surgical procedure can resolve and prevent problems such as ischemic complications in the upper abdominal organs. If the anomaly is congenital, however, the celiac artery has been occluded for a long time and stenosis may not be resolved by division of the MAL. Some surgeons have reported cases of CAS that were resolved by bypass surgery between the CHA or carotid artery and aorta via the saphenous vein [3]. Farma and Hoffman [8] reported 14 cases of celiac artery stenosis due to MAL compression, 11 of which were resolved by MAL division and 3 of which were treated with bypass via the saphenous vein. Nara et al. [3] reported their experience in treating 7 patients with CAS who underwent PD. Two of the 4 patients without arterial reconstruction or preservation showed marked elevation of liver enzymes, and subsequently developed liver abscess. Two patients who underwent arterial reconstruction and 3 patients who showed no decrease in the intrahepatic arterial flow after clamping of the GDA developed no ischemic complications. Only 1 case of CAS was caused by MAL compression. If surgical resolution is unavailable or ineffective, perioperative stent insertion may be helpful for preventing the occlusion of arterial flow. This procedure can be fatal, however, if thrombosis or stent occlusion occurs. Many reports of successful endovascular treatment of patients with CAS caused by artherosclerosis have been published, but Sharafuddin et al. [10] described the risk of stent-crushing due to persistent external compression. Therefore, interventional procedures for these patients must be carefully selected.
The difference between our cases and others published previously is in the attempt to insert the stent through the GDA stump intraoperatively (Fig. 1). After insertion of the stent, the CHA flow disappeared. We assumed that migration or instable location of the stent might cause the formation of thrombosis in and around the stent and the blood flow disappearance. We resolved that problem by performing arterial resection and creating an anastomosis. We also changed the vascular access method from a femoral to a brachial approach in the second case because of acute angulation of the celiac os. By changing the vascular approach, double angulation was changed to single angulation (Fig. 3).
In our institution, the CT angiography has not been performed routinely in patients with pancreatic cancer and we could not detect the possibility of CAS through the preoperative abdominal CT in the aforementioned first case. When we reviewed the preoperative CT images after performing the operation, we could estimate the CAS existence. In the second case, in contrast to the first case, unclear celiac os was found in the preoperative CT and we could plan the postoperative interventional stent insertion. Although the primary intention of stent insertion through the femoral approach failed, stent insertion succeeded by changing the vascular approach from the femoral to the brachial approach.
Based upon our experience, we found it necessary to improve preoperative evaluation and strategies for treating patients who had CAS due to MAL compression. First, communication between the surgeon and radiologist in CT evaluation of celiac stenosis is very important. If the radiologic image shows an advanced collateral vessel or enlarged GDA, the possibility of CAS should be considered even if CAS is not shown definitively. Then, if necessary, performing balloon angioplasty and stenting of the celiac axis would be possible perioperatively. Second, in operative techniques involving MAL division and celiac artery reconstruction, saving the collateral vessels is also important. These techniques may reduce the necessity for additional interventions for CAS. As mentioned previously, however, arterial reconstruction during PD is not essential, because collateral pathways are often developed. However, in patients with malignant tumors, preservation of these collaterals pathways is often impossible or may be undesirable because of the need to ensure surgical curability [3]. Finally, before division, the GDA should be clamped with palpation or the hepatic artery should be assessed by Doppler ultrasound to ensure that there is adequate blood flow to the liver.
Figures and Tables
Fig. 1
A 57-year-old female who received pylorus-resecting PD for pancreatic head cancer, after the resection of gastroduodenal artery (GDA); the flow of common hepatic artery was not detected and celiac axis stenosis was identified intraoperatively. (A) Intervention failed due to acute angulation (full line, arrow) of the celiac orifice. (B) The patient was sent back to operating room, and stent insertion (circle) was performed through GDA stump intraoperatively.
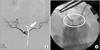
Fig. 2
Postoperative CT image of 1st case. (A) Patent arterial flow of common hepatic artery (arrow) and intrahepatic arteries. (B) Stent located at celiac orifice and showed patent arterial flow to the splenic artery.
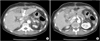
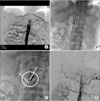
Fig. 3
A 56-year-old male received pylorus-preserving PD for pancreatic head cancer, and loss of hepatic artery flow was detected after gastroduodenal artery ligation. (A) On abdominal arteriography, the flow to the celiac trunk was delayed severely. (B) Approach of the balloon and stent was impossible due to acute angulation (dotted line) of the celiac orifice. (C) Ballooning and stent insertion for stenotic lesion (circle) by the brachial approach (full line) was performed. (D) Arterial flow was improved after the procedure.
References
1. Bron KM, Redman HC. Splanchnic artery stenosis and occlusion. Incidence; arteriographic and clinical manifestations. Radiology. 1969; 92:323–328.
2. Szilagyi DE, Rian RL, Elliott JP, Smith RF. The celiac artery compression syndrome: does it exist? Surgery. 1972; 72:849–863.
3. Nara S, Sakamoto Y, Shimada K, Sano T, Kosuge T, Takahashi Y, et al. Arterial reconstruction during pancreatoduodenectomy in patients with celiac axis stenosis: utility of Doppler ultrasonography. World J Surg. 2005; 29:885–889.
4. Park CM, Chung JW, Kim HB, Shin SJ, Park JH. Celiac axis stenosis: incidence and etiologies in asymptomatic individuals. Korean J Radiol. 2001; 2:8–13.
5. Berney T, Pretre R, Chassot G, Morel P. The role of revascularization in celiac occlusion and pancreatoduodenectomy. Am J Surg. 1998; 176:352–356.
6. Sugae T, Fujii T, Kodera Y, Kanzaki A, Yamamura K, Yamada S, et al. Classification of the celiac axis stenosis owing to median arcuate ligament compression, based on severity of the stenosis with subsequent proposals for management during pancreatoduodenectomy. Surgery. 2012; 151:543–549.
7. Turner KM, Majekodunmi K, Manejwala A, Neschis D, Novak Z, Boutros C. Image findings in celiac artery stenosis due to median arcuate ligament compression: a crucial diagnosis when planning for pancreaticoduodenectomy. J Gastrointest Surg. 2014; 18:638–640.
8. Farma JM, Hoffman JP. Nonneoplastic celiac axis occlusion in patients undergoing pancreaticoduodenectomy. Am J Surg. 2007; 193:341–344.
9. Horton KM, Talamini MA, Fishman EK. Median arcuate ligament syndrome: evaluation with CT angiography. Radiographics. 2005; 25:1177–1182.
10. Sharafuddin MJ, Olson CH, Sun S, Kresowik TF, Corson JD. Endovascular treatment of celiac and mesenteric arteries stenoses: applications and results. J Vasc Surg. 2003; 38:692–698.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download