Abstract
Purpose
Iliac extension of stent-graft during endovascular aneurysm repair (EVAR) increases the incidence of limb occlusion (LO). Hypothetically, adjunctive iliac stent (AIS) could offer some additional protection to overcome this anatomic hostility. But still there is no consensus in terms of effective stent characteristics or configuration. We retrospectively reviewed our center's experience to offer a possible answer to this question.
Methods
Our study included 30 patients (38 limbs) with AIS placed in the external iliac artery (EIA) from January 2010 to December 2013. We classified iliac tortuosity based on anatomic characteristics. AIS's were deployed in EIA with a minimum 5-mm stick-out configuration from the distal edge of the stent-graft.
Results
According to the iliac artery tortuosity index, grade 0, grade 1, and grade 2 were 5 (13.2%), 30 (78.9%), and 3 (7.9%), respectively. The diameter of all AIS was 12 mm, which was as large as or larger than the diameter of the stent-graft distal limb. SMART stents were preferred in 34 limbs (89.5%) and stents with 60-mm length were usually used (89.5%). During a mean follow-up of 9.13 ± 10.78 months, ischemic limb pain, which could be the sign of LO, was not noticed in any patients. There was no fracture, kinking, migration, in-stent restenosis, or occlusion of AIS.
Abdominal aortic aneurysm (AAA) was accompanied by common iliac artery aneurysm (CIAA) in approximately 20% to 30% of patients [12]. CIAA could require extension of the stent-graft limb to the external iliac artery (EIA) in the case of standard bifurcated stent-graft. The risk of iliac limb occlusion (LO) increases in stent-graft limb deployment in EIA [34]. LO of a stent-graft is a serious complication that can lead to amputation; and the prevalence of LO has been reported ranging from 0.7% to 6.4% after endovascular aneurysm repair (EVAR) [35678]. The LO rate of stent-graft limb landed in the EIA was significantly higher in comparison with the common iliac artery (CIA) (15% vs. 3%) [3]. The causes for higher LO are the EIA's smaller caliber, tortuous course and reduction of outflow by covering the internal iliac artery (IIA) [9].
Some papers had reported that the use of adjunctive iliac stent (AIS) with EVAR significantly reduced the risk of LO. The papers mentioned that primary rather than secondary use of AIS was effective with preoperative imaging or completion angiography [410]. Yet, there remains no consensus in terms of effective stent characteristics or configuration. We retrospectively reviewed our center's experience about stent characteristics and configuration.
From January 2010 to December 2013, 98 patients with AAA underwent EVAR in our institution. Among them, CIAA was accompanied with AAA in 35 patients and 55 limbs. This study included 30 patients and 38 limbs requiring extension of the stent-graft limb to the EIA and deployment of AIS.
When a stent-graft iliac limb was needed to extend to the EIA, we always deployed AIS for prevention of LO. All patients were related to the CIAA which required stent-graft placement across the origin of the IIA. When the CIAA was close to or involved the iliac bifurcation or when the CIA was too large to fit the available stent-grafts, we determined to cover IIA. If IIA was not occluded in the preprocedural imaging study, IIA was embolized to prevent type II endoleak. After IIA embolization, stent-grafts were deployed for aneurysm exclusion in the usual manner.
After standard deployment of the endovascular stent-graft, AIS was implanted in EIA. Self-expandable, bare metal stents, such as SMART stent (Cordis Co., Miami, FL, USA), Zilver stent (Cook Inc., Bloominton, IN, USA) and Wall stent (Boston Scientific, Natick, Ma, USA) were used as AIS in our institution. The diameter of all AIS was 12 mm regardless of the diameter of stent-graft distal limb. The AIS was deployed a minimum of 5 mm beyond the distal stent-graft into the EIA, rendering a smooth transition from the stent-graft limb into the native iliac arterial curvature. All of the AIS were placed from the limb to native artery (Fig. 1).
Three-dimensional computed tomographic angiography (3D CTA) using Aquarius, iNtuition Ed ver. 4.4.6 (TeraRecon Inc., Foster City, CA, USA) was examined to plan EVAR and to determine the necessity for iliac limb extension and coverage of the IIA. 3D CTA was performed for review of the aortoiliac anatomy, tortuosity of vessels, and IIA diameter. Iliac artery tortuosity was classified from 0 to 3 by tortuosity index using a 3D workstation (Fig. 2). This data was used to determine the optimal approach for successful catheterization, the size of stent-graft, and the ideal AIS. Follow-up 3D CTA was performed within the 1st postoperative week and then at 6 months and every following year to determine fracture, kinking, migration, in-stent restenosis, and occlusion of AIS as well as the patency of the stent-graft. Physical examination with pulse status and ankle-brachial index were routinely checked with doppler for the assessment of LO.
Between January 2010 and December 2013, 30 patients and 38 limbs requiring extension of the stent-graft limb to the EIA and deployment of AIS at the time of EVAR were reviewed retrospectively. All patients were related to the combined aneurysm of aorta and CIA. Seventeen patients with bilateral CIAAs and 13 patients with unilateral CIAA were included in this study.
Patient demographics and past medical history were listed in Table 1. Mean age of all patients with AIS was 72.13 ± 6.83 years and there were 27 men (90.0%). Thirteen patients (43.3%) had hypertension as comorbidity and 12 patients (40%) had a history of smoking. Procedural characteristics were presented in Table 2. Eight patients (26.7%) were implanted with both AIS's and all underwent additional procedures to prevent pelvic ischemia. Bypass operation from EIA to IIA was performed in 7 patients and an iliac-branched device was used in 1 patient for prevention of pelvic ischemic complications. IIA embolization ipsilateral to AIS was performed in 20 patients to prevent type II endoleak from IIA.
Twenty-seven Zenith (Cook Inc., Bloomington, IN, USA) and 3 Excluder (W.L. Gore & Associates, Sunnyvale, CA, USA) stent-grafts were used. The diameter of all AIS was 12 mm, which was as large as or larger than the diameter of stent-graft distal limb. The diameters of stent-graft distal limbs were 12 mm (28 limbs, 73.8%), 10 mm (9 limbs, 23.7%) and 8 mm (1 limb, 2.6%). SMART stents were preferred in 34 limbs (89.5%) and stents with 60-mm length were used mainly (89.5%) (Table 3). Mean CIA maximum diameter including CIAA was 30.64 mm and mean EIA minimum diameter was 10.97 mm (Table 4). By iliac tortuosity index, grade 1 was found in 30 (78.9%). There was no iliac anatomy as tortuous as grade 3 (Table 5).
During a mean follow-up of 9.13 ± 10.78 months, there was no LO in the patients with AIS. Two patients diagnosed with ruptured AAA at the time of the hospital visit had expired from hypovolemic shock after EVAR. One patient with progressive abdominal pain was endoscopically confirmed with subacute ischemic colitis on the 4th postprocedural day. This patient underwent colon resection and colostomy. However, there were no complications associated with the AIS procedure such as fracture, kinking, migration, in-stent restenosis and occlusion of AIS.
In our study, LO and other complications of AIS procedure didn't happen after AIS deployment with EVAR. This is compatible with previous studies. Oshin et al. [4] compared the group with more aggressive AIS stenting strategy (n = 293) and the group with an ad hoc AIS stenting basis (n = 288). More frequent use of AIS was associated with a concurrent reduction in the rate of LO. Sivamurthy et al. [10] evaluated limb patency with and without AIS. No LO occurred in the group with AIS while there were 13 instances (5.2%) of LO in the group without AIS. Like our results, the studies reported that AIS significantly reduced the risk of LO without complications. AIS protruding from stiff stent-graft to native artery seemed to create a protective radial force to prevent LO. We unexceptionally deployed primary AIS, when stent-graft was extended to EIA. There remains uncertainty regarding the timing of AIS implantation. As EVAR experience increases, there has been a trend toward primary rather than secondary utilization of AIS based on preoperative imaging or completion angiography [4]. Sivamurthy et al. [10] now routinely insert AIS in cases of EIA implantation, as do we.
Although we did not compare self-expandable stents (SES) with balloon expandable stents, SES has been compared in another report [11]. The main purpose of the stent was to offer a smooth transition zone between the stiff stent-graft and the flexible native artery using radial force, which had been demonstrated in atherosclerotic stenotic artery. We believe SES works better for this intention. Different SES's also were used as AIS in our study. Our most commonly used AIS was the SMART stent (89.5%). This stent is an open-cell designed SES that is flexible and resistant to deformation, unlike balloon-expandable stents [1213]. There were no LOs and no complications in the Wall stent group [10]. On the other hand, the use of Zilver stent as AIS for limbs deployed into the EIA did not reduce the rate of limb thrombosis. Three limbs occluded despite the use of AIS in 8 limbs [3]. Insufficient numbers in the studies did not explain clearly that the different results happened although they were also SES's. Because SMART stent, used mainly in our institute, had greater radial resistive force and longitudinal stability, it was believed that SMART stent played an appropriate role as AIS. AIS of 12 mm in diameter, as large as or larger than the diameter of stent-graft distal limb, was safe regardless of different EIA diameter size (8.45–13.10 mm). The configuration of AIS is important for the successful function of AIS. We always place the AIS sticking out at least 5 mm beyond the stent-graft. Since other studies did not make clear statements about the stent configuration, we could not compare whether this policy works in a positive way or not. Iliac tortuosity grade 1 or less occupied most of our study (92.1%) and there was no iliac anatomy as tortuous as grade 3. AIS caused relatively less tortuous iliac artery and never created any kink points in EIA. Because tortuous iliac artery can increase the risk of kinking, fracture, and migration in AIS, iliac tortuosity should be considered thoroughly with AIS installation.
In a few reports, racial differences in the arterial diameter are informed. In an American population, the diameter of the infrarenal aorta was 23 mm in males and 19 mm in females [9]. By Joh et al. [14] analyzing the normal diameter of the abdominal aorta and the bilateral iliac arteries in 1,218 people except for the population diagnosed with AAA, the diameter of the infrarenal aorta was 19.0 mm in males and 17.9 mm in females while the right iliac artery was 11.9 mm and the left iliac artery was 12.7 mm in the Korean population. By analogy with the above listed facts, the Korean populations are exposed to the risk of LO when stent-graft iliac limb was placed in the EIA with EVAR because of the EIA's smaller caliber. The EIA's smaller caliber was known as the risk factor of LO with angulation of the iliac limbs, female gender, arterial dissection and stent-graft extension into the EIA [151617]. More detailed examination is required by preoperative 3D CTA and intraoperative completion angiography in patients with risk factors to prevent LO.
Despite the short follow-up duration (9.13 ± 10.78 months), LO will be less likely to occur because the majority of LO occurred within 6 months [34]. Routine insertion of AIS with limb extension into EIA has limited our ability to interpret the findings of this study without a control group. It may be argued that routine use of AIS is adequate in cases where the limb extends into the EIA. The additional expense and potential risk associated with stent insertion should be considered. By Oshin et al. [4], AIS with limb extension into EIA was selectively performed in cases with a perceived risk of iliac LO based upon either preoperative computed tomography assessment or completion angiography. Routine intravascular ultrasound following stent-graft placement to identify graft limb stenosis [18] and intraoperative 3D rotational angiography [19] can be used to avoid unnecessary AIS.
Our study had some limitations. First, this is a retrospective study with small sample size. Second, this is not a comparative study between 'with AIS' and 'without AIS' because AIS was always deployed when stent-graft was extended to EIA. A larger size and a randomized controlled trial would be needed to evaluate the suitability of AIS.
In conclusion, the installation of AIS after extension of stent-graft to EIA seemed to prevent LO without any procedure-related complications. Primary AIS deployment using 12-mm SES in less tortuous iliac artery should be a good technique to prevent the risk of LO in the Korean population with a small EIA diameter. AIS may be considered as a preventive procedure of LO if stent-graft needs to be extended to EIA during EVAR.
Figures and Tables
Fig. 1
(A) Both iliac stent-graft limbs were deployed on external iliac arteries. The right internal iliac artery was embolized with coils, and adjunctive iliac stents were deployed on both external iliac arteries. (B) Both iliac stent-graft limbs were deployed on external iliac arteries. The right internal iliac artery was embolized with coils, and adjunctive iliac stents were deployed on both external iliac arteries.
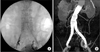
Fig. 2
(A) Measuring the iliac tortuosity index. The iliac tortuosity index was defined by dividing L1 by L2, where L1 (A and B) was the distance along the central lumen line between the common femoral artery and the aortic bifurcation, and L2 (B) was the straight-line distance from the common femoral artery and the aortic bifurcation. In this case, the iliac tortuosity index was 1.36 (197 of 145). (B) Measuring the iliac tortuosity index. The iliac tortuosity index was defined by dividing L1 by L2, where L1 (A and B) was the distance along the central lumen line between the common femoral artery and the aortic bifurcation, and L2 (B) was the straight-line distance from the common femoral artery and the aortic bifurcation. In this case, the iliac tortuosity index was 1.36 (197 of 145).

References
1. Hobo R, Sybrandy JE, Harris PL, Buth J. EUROSTAR Collaborators. Endovascular repair of abdominal aortic aneurysms with concomitant common iliac artery aneurysm: outcome analysis of the EUROSTAR Experience. J Endovasc Ther. 2008; 15:12–22.
2. Armon MP, Wenham PW, Whitaker SC, Gregson RH, Hopkinson BR. Common iliac artery aneurysms in patients with abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 1998; 15:255–257.
3. Conway AM, Modarai B, Taylor PR, Carrell TW, Waltham M, Salter R, et al. Stent-graft limb deployment in the external iliac artery increases the risk of limb occlusion following endovascular AAA repair. J Endovasc Ther. 2012; 19:79–85.
4. Oshin OA, Fisher RK, Williams LA, Brennan JA, Gilling-Smith GL, Vallabhaneni SR, et al. Adjunctive iliac stents reduce the risk of stent-graft limb occlusion following endovascular aneurysm repair with the Zenith stent-graft. J Endovasc Ther. 2010; 17:108–114.
5. Hobo R, Buth J. EUROSTAR collaborators. Secondary interventions following endovascular abdominal aortic aneurysm repair using current endografts. A EUROSTAR report. J Vasc Surg. 2006; 43:896–902.
6. EVAR trial participants. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet. 2005; 365:2179–2186.
7. EVAR trial participants. Endovascular aneurysm repair and outcome in patients unfit for open repair of abdominal aortic aneurysm (EVAR trial 2): randomised controlled trial. Lancet. 2005; 365:2187–2192.
8. Prinssen M, Verhoeven EL, Buth J, Cuypers PW, van Sambeek MR, Balm R, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2004; 351:1607–1618.
9. Ouriel K, Green RM, Donayre C, Shortell CK, Elliott J, DeWeese JA. An evaluation of new methods of expressing aortic aneurysm size: relationship to rupture. J Vasc Surg. 1992; 15:12–18.
10. Sivamurthy N, Schneider DB, Reilly LM, Rapp JH, Skovobogatyy H, Chuter TA. Adjunctive primary stenting of Zenith endograft limbs during endovascular abdominal aortic aneurysm repair: implications for limb patency. J Vasc Surg. 2006; 43:662–670.
11. Shin ES, Garcia-Garcia HM, Okamura T, Wykrzykowska JJ, Gonzalo N, Shen ZJ, et al. Comparison of acute vessel wall injury after self-expanding stent and conventional balloon-expandable stent implantation: a study with optical coherence tomography. J Invasive Cardiol. 2010; 22:435–439.
12. Dyet JF, Watts WG, Ettles DF, Nicholson AA. Mechanical properties of metallic stents: how do these properties influence the choice of stent for specific lesions? Cardiovasc Intervent Radiol. 2000; 23:47–54.
13. A comparison of balloon- and self-expanding stents. Minim Invasive Ther Allied Technol. 2002; 11:173–178.
14. Joh JH, Ahn HJ, Park HC. Reference diameters of the abdominal aorta and iliac arteries in the Korean population. Yonsei Med J. 2013; 54:48–54.
15. Carroccio A, Faries PL, Morrissey NJ, Teodorescu V, Burks JA, Gravereaux EC, et al. Predicting iliac limb occlusions after bifurcated aortic stent grafting: anatomic and device-related causes. J Vasc Surg. 2002; 36:679–684.
16. Woody JD, Makaroun MS. Endovascular graft limb occlusion. Semin Vasc Surg. 2004; 17:262–267.
17. Carpenter JP, Neschis DG, Fairman RM, Barker CF, Golden MA, Velazquez OC, et al. Failure of endovascular abdominal aortic aneurysm graft limbs. J Vasc Surg. 2001; 33:296–302.
18. Amesur NB, Zajko AB, Orons PD, Makaroun MS. Endovascular treatment of iliac limb stenoses or occlusions in 31 patients treated with the ancure endograft. J Vasc Interv Radiol. 2000; 11:421–428.
19. Nordon IM, Hinchliffe RJ, Malkawi AH, Taylor J, Holt PJ, Morgan R, et al. Validation of DynaCT in the morphological assessment of abdominal aortic aneurysm for endovascular repair. J Endovasc Ther. 2010; 17:183–189.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download