Abstract
Purpose
To analyze the risk factors for postoperative lymphocele, for predicting and preventing complications.
Methods
We evaluated 92 kidney transplant recipients with multidetector CT (MDCT) at 1-month posttransplantation. From admission and 1-month postoperative records, data including diabetes, dialysis type, immunosuppressant use, steroid pulse therapy, and transplantation side were collected. Lymphocele volume was measured with 3-dimensional reconstructed, nonenhanced MDCT at one month postoperatively. The correlations between risk factors and lymphocele volume and between risk factors and symptomatic lymphocele (SyL) were analyzed. The cutoff was calculated by using the receiver operating characteristic (ROC) curve for SyL volume.
Results
Among 92 recipients, the mean volume was 44.53 ± 176.43 cm3 and 12 had SyL. Univariable analysis between risk factors and lymphocele volume indicated that donor age, retransplantation, and inferiorly located lymphocele were statistically significant. The ROC curve for SyL showed that 33.20 cm3 was the cutoff, with 83.3% sensitivity and 93.7% specificity. On univariable analysis between risk factors and SyL, steroid pulse, inferiorly located lymphocele, and >33.20 cm3 were statistically significant. Multivariable analysis indicated that steroid pulse, >33.20 cm3, and serum creatinine level at one month were significant factors.
Conclusion
Risk factors including donor age, retransplantation, steroid pulse therapy, and inferiorly located lymphocele are important predictors of large lymphoceles or SyL. In high-risk recipients, careful monitoring of renal function and early image surveillance such as CT or ultrasound are recommended. If the asymptomatic lymphocele is >33.20 cm3 or located inferiorly, early interventions can be considered while carefully observing the changes in symptoms.
Postoperative lymphocele formation in kidney transplant recipients involves the collection of fluid, particularly lymphatic fluid, around the renal allograft. The incidence of this complication is reported to be 12%–40% [1]. In many cases, the lymphocele disappears over time, without causing any symptoms. Therefore, lymphoceles are often found incidentally during regular ultrasonography or nonenhanced CT. In cases of symptomatic lymphocele (SyL), the most common symptom is graft dysfunction; however, various other symptoms also develop, such as perigraft distension, ureteric obstruction, leg swelling, deep vein thrombosis, urinary frequency, lower abdominal pain, or fever [23]. Lymphoceles appear most commonly at 2–6 weeks after surgery [4]; rare cases of lymphoceles presenting 6 years after the trauma or infection have been reported.
Many studies have been performed on SyL that is diagnosed while determining the causes of the symptoms. However, few studies have been conducted on lymphocele overall, including the asymptomatic type. Clinical studies play an important role in deciding proper treatment and assessing prognosis. Through the quantitative analysis and symptom-correlation analysis of postoperative lymphocele by using 3-dimensional (3D) multidetector CT (MDCT) reconstruction, the proper surveillance can be planned and the proper timing of intervention can be decided depending on the degree of risk in kidney transplant recipients.
The present study was approved by the Investigation Review Committee (No. ED15067). We retrospectively analyzed 92 patients who had received a kidney transplant from January 2012 to December 2014. Of these patients, 61 received a living-donor kidney transplant through hand-assisted laparoscopic surgery; 31 patients received a kidney from a deceased donor.
The retroperitoneal approach was used that involved dissection of the iliac vein, artery, and bladder dome through a J-shaped skin incision, known as "Gibson incision." During vessel dissection, the surrounding tissues including lymphatics were tied routinely. Vein and artery anastomosis was performed in an end-to-side fashion between the graft renal vessel and the recipient's external iliac vessel. Ureter anastomosis was performed through ureteroneocystostomy with an antireflux technique. A double-J stent was placed from the graft hilum to the recipient's bladder to prevent urinary complications. In all recipients, a Jackson-Pratt drain was routinely placed in the inferior space from the posterior side of the graft and the hilum. The recipients were usually discharged from the hospital between postoperative days 10 and 14.
The immunosuppressant chosen for induction therapy was antithymoglobulin or basiliximab. The combination of a calcineurin inhibitor, mycophenolate mofetil, and corticosteroids was used mainly for maintaining immunosuppression. In four recipients, sirolimus was used in place of mycophenolate mofetil. In contrast with the conventional steroid regimen, early steroid withdrawal within 8 days was done for 7 recipients.
On the basis of the date of admission for surgery, we retrospectively analyzed many risk factors in the patients, including diabetes, body mass index, dialysis type, retransplantation history, and ABO incompatibility. From medical records derived from the 1-month postoperative follow-up, we collected data on immunosuppressant use, plasmapheresis, steroid pulse therapy, side of the donor's nephrectomy, side of the recipient's transplant, multiplicity of the graft renal artery, serum creatinine levels, and mean weight change at one month postoperatively. Mean weight change was estimated as follows: (weight at 1 month postoperatively – weight at admission)/(weight at admission) × 100, and expressed as a percentage. This computation has been used to assess body composition change in sports medicine [5].
The criteria for determining SyL comprised patient complaints and hydroureteronephrosis with postoperative lymphocele in the evaluation of postoperative images. Complaints included abdominal pain and swelling around the allograft and lower-extremity swelling (a symptom of venous compression). After the aspirated perigraft fluid was analyzed, percutaneous drainage or fenestration, urinoma, abscess, and hematoma were excluded as criteria based on creatinine levels, fluid analysis results, and culture results.
At 4–6 weeks postoperatively, after an MDCT scan was performed, the double-J catheter was removed. By using an MDCT scan taken 1 month postoperatively, regardless of symptoms, the quantitative volume of lymphocele was measured in the D reconstruction images (Fig. 1). To obtain 3D volumetry, CT examinations of the abdomen were performed with a 128-slice MDCT scanner (Definition AS Plus; Siemens Healthcare, Forchheim, Germany) without contrast agent administration. After the scan, an automatic raw-data analysis tool (Syngo and Somaris; Siemens Medical Solutions, Erlangen, Germany) was used to reconstruct the transverse CT data. Next, 2 reviewers (HJ, SHH) assessed the analysis of the abdominal CT images by using a commercial software (TeraRecon iNtuition, TeraRecon, Foster City, CA, USA). By using the free region of interest for hand drawing, the lymphocele images were manually collected within the overlay images, and the lymphocele volume was automatically assessed in cubic centimeters and expressed on the 3D image.
Continuous data are expressed as the mean ± standard deviation. The univariable analysis used to correlate lymphocele volume with various risk factors at 1 month after the operation was performed by using Spearman correlation analysis and the Mann-Whitney U-test in view of the nonparametric group. According to the above definition, all patients with lymphocele were classified into either the symptomatic or asymptomatic group. By using a receiver operating characteristic (ROC) curve for lymphocele volume in symptomatic cases, the cutoff value of lymphocele volume was calculated with higher than 80% sensitivity and 90% specificity. This univariable analysis to determine the correlation between risk factors and SyL was assessed by using Pearson chi-square analysis for 2 categorical variables and linear-by-linear association analysis for continuous variables, after dividing by interval. For multivariable analysis, a multivariable mixed-regression analysis was used. All statistical analyses were performed with SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). A P-value of <0.05 was considered statistically significant.
Among the 107 kidney transplantations performed from January 2012 to December 2014, we excluded 15 patients, including four who underwent kidney transplantation through a peritoneal approach owing to simultaneous pancreas kidney transplantation or concomitant nephrectomy, 2 who died within 1 month of the operation, 6 who were lost to CT follow-up, 1 child who did not undergo CT follow-up owing to radiation concerns, and 2 foreigners who returned to their own country within 1 month postoperatively.
Of the 92 kidney recipients included in the study, the mean age was 42.80 ± 12.46 years and 69 (75.0%) were men. The reported baseline risk factor characteristics included 14 patients (15.2%) with ABO incompatibility, 6 (6.5%) with retransplantation, 24 (26.1%) with plasmapheresis, 13 (14.1%) with steroid pulse therapy, and 31 (33.7%) with an inferiorly located lymphocele. Twenty-four recipients with plasmapheresis included 14 with ABO incompatibility, 3 with preformed donor-specific antibody, 3 with high panel reactive antibody, 2 with antibody-mediated rejection, 1 with hemolytic uremic syndrome, and 1 with focal segment glomerulosclerosis. The mean graft weight was 182.26 ± 34.08 g. The mean lymphocele size, as seen on CT 3D volumetry, was 44.53 ± 176.43 cm3 (Table 1). A total of 12 recipients (13.0%) had SyL (Table 2) and eventually required medical intervention: 3 (3.2%) required aspiration, 4 (4.3%) required percutaneous drainage, and 5 (5.4%) required laparoscopic fenestration.
The univariable analysis between risk factors and lymphocele volume indicated that donor age, retransplantation, and inferiorly located lymphocele were statistically significant factors. In addition, steroid pulse therapy and graft weight seemed to be related to the size of lymphocele, but without statistical significance (Table 3). In the ROC curve for SyL, 33.20 cm3 was the cutoff volume, with a sensitivity of 83.3% and a specificity of 93.7% (Fig. 2).
In the univariable analysis correlating SyL with its risk factors, steroid pulse therapy, lymphocele location, and >33.20 cm3 lymphocele volume were found to be statistically significant factors (Table 4). In addition, retransplantation and dialysis type seemed to be related to the occurrence of SyL, but with no statistical significance (Table 4). The analysis of the locations of SyL indicated that the lymphocele had a superolateral location in 3 of 16 cases (18.7%), hilar location in 2 of 11 cases (18.1%), and inferior location in 10 of 31 cases (32.2%). In a multivariable analysis of SyL, a volume of >33.20 cm3, serum creatinine level at 1 month postoperatively, and steroid pulse therapy were all shown to be statistically significant factors (Table 5).
Our study identified high-risk factors that influence the volume and symptoms of postoperative lymphoceles. In fact, the volume is also a risk factor for SyL. According to the risk factor analysis, our study presented clinical suggestions for these high-risk recipients concerning additional surveillance and timing of intervention. Much research has been conducted on SyL diagnosed while determining the causes of the symptoms including graft dysfunction. However, little information is available on lymphocele overall, including asymptomatic cases. Our study is significant in terms of presymptomatic risk analysis and clinical prevention.
Concerning SyL, Zagdoun et al. [6] reported on the symptoms of patients with complicated lymphocele, including incidental findings in 29%, abdominal swelling in 25%, liquid leakage from wound in 20.8%, acute obstructive renal failure in 18.7%, and edema of the ipsilateral lower extremity in 4.2%. The symptoms of SyL in this study include abdominal discomfort, wound discharge, elevated creatinine, hydroureter, and leg swelling (Table 2). These symptoms were not discovered incidentally in the recipients, and required intervention because of the relevance of the symptoms to graft conditions including function, infection, and venous drainage.
Most cases of lymphocele in kidney recipients derive from the recipients' iliac lymphatic leakages during vessel dissection [2]. Lymphatic leakages also occur in the donor's graft, such as at the renal hilum and surface [78]. Many factors including multiple renal arteries, right-side procurement [9], obesity [10], diabetes [3], and acute rejection [11] were reported as risk factors for lymphocele development. Although sirolimus has been reported to contribute to lymphocele formation [12], no significant relationship was found in this study. A study of large volumes is needed.
Many clinicians have their own methods for measuring lymphocele size and their own criteria for diagnosing SyL. Adani et al. [13] reported that a lymphocele with a volume of <50 cm3 and a diameter of <3 cm can be sufficiently treated with conservative management. Ulrich et al. [3] maintain that if the lymphocele diameter is 3–5 cm, percutaneous drainage should be selected, and if the diameter exceeds 5 cm, laparoscopic unroofing should be used. SyL has been suggested to involve a lymphocele diameter of >2.5 cm [3] or >4 cm [14]. Krol et al. [15] defined the volume to be >140 cm3 as seen on ultrasonography. Our study also proposed that a lymphocele that is >33.20 cm3 or located inferiorly is likely to cause symptoms.
MDCT surveillance at one-month follow-up is preferred in our center. CT is a more accurate modality for localizing the collection and determining its relation to important surrounding structures than is ultrasound [16]. In ultrasound scanning, many subjective factors depend on the operator, such as the scanning angle, depth, and interference from adjacent structures. Considering that lymphocele usually occurs at 2–6 weeks after surgery [4], MDCT imaging at 1 month postoperatively has various merits, such as early detection of asymptomatic lymphocele, detection of concomitant wound problems, and assessment of hydroureter.
In conclusion, risk factors including donor age, retransplantation, steroid pulse therapy, and inferiorly located lymphocele are important predictors of large lymphocele volumes or SyL. In high-risk recipients, careful monitoring of renal function and early image surveillance such as CT or ultrasonography are recommended. If the asymptomatic lymphocele is >33.20 cm3 or located inferiorly, early interventions can be considered while carefully observing the changes in symptoms.
Figures and Tables
Fig. 1
An example multidetector CT image of a postoperative lymphocele in a kidney transplant recipient. (A) Axial image (white arrow). (B) Three-dimensional reconstruction and automatic volume assessment.
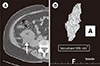
Fig. 2
Receiver operating characteristic curve and cutoff lymphocele volume for a symptomatic lymphocele. AUC, area under the curve.
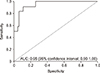
Table 1
Baseline risk factor characteristics for postoperative lymphocele in kidney transplant recipients

Table 3
Univariable analysis showing correlations between lymphocele amounts and risk factors in kidney recipients, 1 month postoperatively

References
1. Zietek Z, Sulikowski T, Tejchman K, Sienko J, Janeczek M, Iwan-Zietek I, et al. Lymphocele after kidney transplantation. Transplant Proc. 2007; 39:2744–2747.
2. Ebadzadeh MR, Tavakkoli M. Lymphocele after kidney transplantation: where are we standing now? Urol J. 2008; 5:144–148.
3. Ulrich F, Niedzwiecki S, Fikatas P, Nebrig M, Schmidt SC, Kohler S, et al. Symptomatic lymphoceles after kidney transplantation - multivariate analysis of risk factors and outcome after laparoscopic fenestration. Clin Transplant. 2010; 24:273–280.
4. Thompson TJ, Neale TJ. Acute perirenal lymphocele formation 8 years after renal transplantation. Aust N Z J Surg. 1989; 59:583–585.
5. Chajes V, Biessy C, Ferrari P, Romieu I, Freisling H, Huybrechts I, et al. Plasma elaidic acid level as biomarker of industrial trans fatty acids and risk of weight change: report from the EPIC study. PLoS One. 2015; 10:e0118206.
6. Zagdoun E, Ficheux M, Lobbedez T, Chatelet V, Thuillier-Lecouf A, Bensadoun H, et al. Complicated lymphoceles after kidney transplantation. Transplant Proc. 2010; 42:4322–4325.
7. Gomes AS, Scholl D, Feinberg S, Simmons RL, Amplatz K. Lymphangiography and ultrasound in management of lymphoceles. Urology. 1979; 13:104–108.
8. Sollinger HW, Starling JR, Oberley T, Glass NR, Belzer FO. Severe “weeping” kidney disease after transplantation: a case report. Transplant Proc. 1983; 15:2157–2160.
9. Chedid MF, Muthu C, Nyberg SL, Lesnick TG, Kremers WK, Prieto M, et al. Living donor kidney transplantation using laparoscopically procured multiple renal artery kidneys and right kidneys. J Am Coll Surg. 2013; 217:144–152.
10. Singh D, Lawen J, Alkhudair W. Does pretransplant obesity affect the outcome in kidney transplant recipients? Transplant Proc. 2005; 37:717–720.
11. Khauli RB, Stoff JS, Lovewell T, Ghavamian R, Baker S. Post-transplant lymphoceles: a critical look into the risk factors, pathophysiology and management. J Urol. 1993; 150:22–26.
12. Nashan B, Citterio F. Wound healing complications and the use of mammalian target of rapamycin inhibitors in kidney transplantation: a critical review of the literature. Transplantation. 2012; 94:547–561.
13. Adani GL, Baccarani U, Bresadola V, Lorenzin D, Montanaro D, Risaliti A, et al. Graft loss due to percutaneous sclerotherapy of a lymphocele using acetic acid after renal transplantation. Cardiovasc Intervent Radiol. 2005; 28:836–838.
14. Giuliani S, Gamba P, Kiblawi R, Midrio P, Ghirardo G, Zanon GF. Lymphocele after pediatric kidney transplantation: incidence and risk factors. Pediatr Transplant. 2014; 18:720–725.
15. Krol R, Kolonko A, Chudek J, Ziaja J, Pawlicki J, Mały A, et al. Did volume of lymphocele after kidney transplantation determine the choice of treatment modality? Transplant Proc. 2007; 39:2740–2743.
16. Abou-Elela A, Reyad I, Torky M, Meshref A, Morsi A. Laparoscopic marsupialization of postrenal transplantation lymphoceles. J Endourol. 2006; 20:904–909.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download