Abstract
Purpose
In this study, we introduced a novel technique, the pancreaticojejunostomy (PJ), which uses only two transpancreatic sutures with buttresses (PJt), and compared the surgical outcomes with previously used methods, especially for hard pancreases.
Methods
A total of 101 patients who underwent pancreaticoduodenectomy with hard pancreases were enrolled and divided into 3 groups according to the method of pancreaticoenteric anastomosis: 30 patients (29.7%) underwent the conventional dunking method (Du), 31 patients (30.7%) underwent pancreaticogastrostomy using transpancreatic sutures (PGt) and 40 patients (39.6%) underwent PJ using transpancreatic sutures (PJt). The surgical outcomes were compared according to the type of anastomosis to analyze the feasibility and ease of each technique.
Results
The overall operative time was shorter in the PJt group (325.1 ± 63.8 minutes) than in the PGt group (367.3 ± 70.5 minutes) or the Du group (412.0 ± 38.2 minutes, P < 0.001). In terms of pancreaticoenteric anastomosis time, it was also shorter in the PJt group (10.3 ± 3.5 minutes) than in the Du group (20.7 ± 0.7 minutes) or the PGt group (16.8 ± 5.4 minutes, P = 0.005). Significant postoperative pancreatic fistula (POPF) developed in 2 cases (6.7%) in the Du group, whereas there were no POPF cases in the PGt or PJt groups (P = 0.086). Overall postoperative morbidities occurred in 31 cases (30.7%), and there were no significant differences among the 3 groups (P = 0.692).
Pancreaticoenteric anastomosis is one of the most important stages of pancreaticoduodenectomy (PD) procedures and often determines the overall outcomes of the surgery. It is quite different from other PD anastomoses, such as choledochojejunostomy, gastrojejunostomy, or duodenojejunostomy, for several reasons. First, the texture of the pancreas is commonly fragile, and this property creates some difficulties avoiding pancreatic laceration during suturing and might lead to pancreatic leakage. Second, the pancreas is fixed to the retroperitoneum, whereas the bowel is freely movable; therefore, pancreaticoenteric anastomosis might be less secure and easier to disrupt than other anastomoses between hollow visceral organs. Third, the dissection plane is usually near major vascular structures, such as the superior mesenteric artery, superior mesenteric vein (SMV), or the portal vein, and also adjacent to various organs, such as the stomach or duodenum, which makes performing the anastomosis more difficult.
Pancreases with a hard texture are thought to have a very low risk of postoperative pancreatic fistula (POPF), which results from anastomotic disruption. This complication is caused by the obstruction of the pancreatic duct by tissue fibrosis, which is one of the characteristics of hard pancreases. Moreover, the tissue strength without distortion or shattering against the shear stress of the needle (suture-holding capacity, SHC) is usually increased in hard pancreas cases, which is helpful for reducing the risk of POPF [1]. Therefore, improvements in technical complexity or difficulty have begun to emerge as a key aspect of pancreaticoenteric anastomosis rather than the type of anastomosis, especially for hard pancreas cases, which have a low risk of POPF.
In our institution, the authors previously proposed pancreaticogastrostomy (PG) using two transpancreatic sutures with buttresses (PGt) in 2010 [2], and we have tried to modify and develop another method to establish the safety and simplicity of pancreaticoenteric anastomosis, especially for hard pancreases. In this study, we introduced a novel technique, the pancreaticojejunostomy (PJ), which uses only two transpancreatic sutures with buttresses (PJt), and compared the surgical outcomes with the conventional dunking method (Du) and the previously described PGt method particularly for hard pancreases.
From March 2006 to March 2015, 231 patients with periampullary lesions underwent PD. All surgical procedures were performed by three specialized pancreas surgeons at our institution. Conventional end-to-end PJ (dunking method) has been the most common PD method performed by the three surgeons; however, surgeon Hong introduced the PGt method in May 2010. Using this method, the anastomosis is completed with two transpancreatic sutures with buttresses on both the upper and lower edges of the implanted pancreas into the stomach through the retracted anterior gastrostomy (Fig. 1) [2], and this technique has been consecutively applied in pancreaticoenteric anastomoses after PD by surgeon Hong In January 2013, the PJt method, which is a modification of the previous PGt method, was developed, and it has been performed only in hard pancreas cases. The hardness of the pancreas is estimated by the surgeon during the operation and is also more objectively based on a durometer measurement of the pancreas hardness using the same method described in previous reports (Fig. 2) [34]. (We considered the pancreas hard when the durometer level was greater than 40 shore units, as described in a previous report.)
Data for all recruited cases were retrospectively reviewed and compared according to the types of pancreaticoenteric anastomosis. Because the PJt has been applied only in hard pancreas cases, in this study, we only enrolled cases in which the conventional Du or PGt were performed with hard pancreases. Among the cases, the patients who underwent any concurrent operation, such as hepatectomy or colectomy, were excluded, and some cases that involved pancreaticoenteric anastomoses, such as duct-to-mucosa PJ or binding PJ, were also excluded from this study. As a result, a total of 101 cases were included and categorized into three groups according to the type of pancreaticoenteric anastomosis: the Du group was performed on 30 patients, the PGt method (the PGt group) was performed on 31 patients, and the PJt method (the PJt group) was performed on 40 patients. All medical data were prospectively collected, including demographics (age, sex, body mass index, pathology, American Society of Anesthesiologists class, tumor characteristics), operative results (overall operative time, pancreaticoenteric anastomosis time, estimated blood loss, intraoperative transfusion) and postoperative outcomes (length of postoperative hospital stay, the start of soft diet, postoperative complications, and mortality).
In the Du group, end-to-end dunking anastomosis was performed as described in the report by Batignani et al. [5]. In the PGt group, PG using two transpancreatic sutures with buttresses was performed as described in a previously published report [2]. In the PJt group, PJ using two transpancreatic sutures with buttresses was performed as described in the next paragraph. After the pancreaticoenteric anastomosis, end-to-side choledocojejunostomy and end-to-side gastrojejunostomy or duodenojejunostomy were performed to establish the continuity of the digestive tract. After completing the reconstruction, we used three closed-suction drains; one was located near the hepaticojejunostomy, and the other two drains were placed around the pancreaticoenterostomy site. Schematic diagrams of the PGt and PJt procedures after the completion of reconstruction are shown in Fig. 3.
After the resection of the pancreas head and duodenum was completed and the specimen was retrieved, approximately 3–4 cm of the length of the pancreas remnant stump was dissected away from the splenic vein and retroperitoneal attachments. Small vessel branches that were located between the pancreas and the SMV or splenic vein were dissected with ultrasound scissors. The location of the main pancreatic duct was identified with a probe, and then, a short plastic stent was inserted at least 4 cm into the duct to avoid the compression of the duct during the ligation of the transpancreatic sutures and to prevent accidental suturing of this duct during the pancreaticoenteric anastomosis. Approximately 3 cm of the length of the stent was left on the cut surface, and a suture was applied to fix the stent to the duct. Two stay sutures on both corners of the pancreas stump were placed to provide traction for the pancreas stump into the jejunal lumen. After preparing the pancreas stump, at least 2 cm of the mucosal layer of the jejunum loop from the cut edge was cauterized with an Argon beam to prevent mucosal secretions. A small hole was made in the jejunum on the antimesenteric side, where the hepaticojejunostomy would be performed, and a Kelly clamp was inserted into the hole to grasp the stay sutures on the pancreas. The operator gently pulled the two stay sutures with the Kelly clamp, and then, the pancreatic stump was brought into the jejunum approximately 3–4 cm and implanted. Next, two transpancreatic sutures with buttresses were placed on both the upper and lower borders of the implanted pancreas through the jejunal limb that covered the pancreas (Fig. 4). This suturing required a pair of 2-0 monofilament polypropylene threads with the specified straight needle at each end (2-0 Prolene, Ethicon Inc., Somerville, NJ, USA) and four buttresses (TFE Polymer Pledget, Ethicon Inc.), which is similar to the PGt method except for the type of threads used (PGt uses 4-0 monofilament polypropylene threads with a straightened needle). First, the suture was passed directly from the anterior surface of the covered jejunal limb, and then, it penetrated the full thickness of the implanted pancreas at a level 2 cm below the cut surface, from the ventral surface to the dorsal surface; next, it passed through the posterior surface of the covered jejunal limb. After this step, the buttresses were inserted through each needle, and the anastomosis was completed with a knot that included the entire jejunal wall and the implanted pancreas. In this method, we did not place any additional reinforcement sutures on the anastomosis line.
A nasogastric tube was kept in place until postoperative day 5 to protect the pancreaticogastric anastomosis from unpredicted gastric distension in the PGt group. With the Du or the PJt, the drains were removed as soon as the drainage was less than 100 mL per day. All patients were given parenteral nutritional support beginning the day after the surgery if their vital signs were stable, and this support was continued until oral feeding could be restarted. On postoperative day 5, patients were routinely evaluated by abdomen CT scans, and if there was no evidence of anastomosis leakage based on both the CT scan and clinical signs, then oral feeding was resumed. Subcutaneous somatostatin therapy was not administered routinely during the postoperative period unless definite evidence of leakage was found. In each case, the overall operative time was defined as the duration from skin incision to closure. To compare the characteristics of each anastomosis type among the 3 groups, the pancreas anastomosis time was specified as the time from the completion of the resection to the completion of the pancreaticoenteric anastomosis with knots, and the time that was not related to the surgery, such as irrigation, was excluded. The overall operative time and the pancreatic anastomosis time were accurately estimated through the review of operative records and video logs.
According to the International Study Group of Pancreatic Fistula (ISGPF) definition [6], POPF is defined as the drainage of any significant volume on postoperative day 3 or later with an amylase concentration of more than 3 times the serum amylase concentration. Each POPF is classified as grades A, B, or C based on the ISGPF criteria as follows: grade A, POPF is clinically stable and requires no treatment or admission; grade B, POPF shows fluid collection on CT scan and may require treatment and/or readmission; and grade C, POPF requires treatment or reoperation, and sepsis or infection may be present [7]. In this study, only grade B or C POPFs were considered clinically significant POPFs because there were no differences between cases with grade A POPFs and without fistulas in terms of clinical findings and progression [89]. Drains were kept in place for at least 5 days after surgery, and the properties and volume of the drained fluid were recorded. The drains were removed after the amylase concentration of the drainage gradually decreased and there was no sign of POPF or intraabdominal infection. Delayed gastric emptying (DGE) is defined as the inability to return to a normal diet by postoperative day 10, or the need to maintain nasogastric intubation or reinstate the intubation [1011]. Postoperative mortality was defined as mortality that developed within 30 days of the operation or during the same hospital stay as the surgery. Patients returned to the outpatient department on the seventh day after discharge, and their general condition was evaluated.
The chi-square test was used for categorical data using frequency distributions and percentages. For continuous variables, the student t-test was used to compare the outcomes between the two groups, and one-way analysis of variance was performed to compare the results among 3 groups. Descriptive statistics were recorded as the means ± standard deviation. The 95% confidence interval of the difference in proportions was calculated, and the test for statistical significance was two sided with a level of significance of 0.05.
In the current study, 57 males (56.4%) and 44 females (43.6%) were included with a mean age of 65.1 ± 8.9 years. Patients underwent one of the 2 types of PD: Whipple's operation (36 patients, 35.6%) or pylorus-preserving pancreaticoduodenectomy (PPPD) (65 patients, 64.4%). The patient demographic findings and the pathologic diagnoses of tumors are shown in Table 1. In the current study, distal common bile duct (CBD) cancer was the most common diagnosis (40 cases, 39.6%), followed by pancreas head cancer (22 cases, 21.8%). We preoperatively performed a percutaneous transhepatic biliary drainage (PTBD) or endoscopic retrograde biliary drainage (ERBD) when the patient showed signs of cholangitis or the obstructive jaundice was severe (a level of total bilirubin in serum >10.0 mg/dL), and preoperative drainage was performed in 37 cases (36.6%) without a significant difference among the three groups (P = 0.198).
The overall operative time was 412.0 ± 38.2 minutes in the Du group, 367.3 ± 70.5 minutes in the PGt group and 325.1 ± 63.8 minutes in the PJt group with a significant difference among the three groups (P < 0.001). The pancreas anastomosis time also showed a significant difference among the 3 groups: 20.7 ± 0.7 minutes in the Du group, 16.8 ± 5.4 minutes in the PGt group and 10.3 ± 3.5 minutes in the PJt group (P = 0.005). The overall operative time and the pancreaticoenteric anastomosis time in the PJt group were significantly shorter than in the Du group or the PGt group. The mean length of postoperative hospital stay and the time to the start of soft diet showed no significant differences among the three groups.
No cases of POPF were observed in the PGt or PJt groups, whereas 2 cases of POPF developed in the Du group (6.7%); one case was grade B, and the other case was grade C POPF. In each case, ultrasound-guided drainage was performed, and antibiotic management with parenteral nutrition was used. Other than POPF, other postoperative complications developed in 31 patients (30.7%): 11 patients (36.7%) in the Du group, 9 patients (29.0%) in the PGt group, and 11 patients (27.5%) in the group PJt group. Details of the overall postoperative morbidities are presented in Table 2, and there were no significant differences among the three groups for each group of complications except atelectasis (P = 0.048); 5 cases of major complications were observed in this study without significant differences (3 cases, 10.0% in the Du group, 2 cases, 6.5% in the PGt group, and no cases in the PJt group, P = 0.159). They included 1 DGE, 1 ileus and 3 postoperative bleeding. Among the cases of postoperative bleeding, one patient in the Du group underwent surgical exploration with achievement of bleeding control, and the other two patients in the PGt group were successfully managed with an endoscopic intervention that coagulated the bleeding focus at the PG anastomosis site. Postoperative mortality developed in 1 case (1.0%) in this study, and the cause of death was sepsis resulting from severe POPF in the Du group.
Hard pancreases are generally accepted as having a low risk of POPF due to the following characteristics. First, the fibrotic changes in the tissue, which are a common finding in hard pancreases, obstruct the minor ducts at the cut-surface of the pancreas, and this could help reduce POPF by blocking the secretions from these minor ducts. Second, the firm nature of the pancreas helps to increase the SHC of the pancreatic tissue itself, and this prevents parenchymal tearing by the suture or tying during the anastomosis. These unique characteristics of hard pancreases help surgeons reduce the chance of POPF and also reduce the worry over choosing the type of anastomosis, whether the conventional duct-to-mucosa method, invagination PJ or even PG. Instead, the technical ease and feasibility of the anastomosis is emphasized in the case of hard pancreases. The difficulty of handling the anastomosis could result in technical errors, affect the stability of the anastomosis or even worsen the stress on the operator. At our institution, surgeon Hong has performed PJt for pancreaticoenteric anastomosis, which is a modification of the PGt for hard pancreases, since January 2013, after ensuring the safety and feasibility of the method through animal experiments using dogs.
Recently, several randomized controlled trials were published describing the improved safety of PG over PJ [1213], and we also agree that the PG could be more effective at reducing the risk of POPF compared to PJ. Nevertheless, we have selectively performed PJt in hard-textured pancreases for several reasons. First, with PG anastomosis, gastric acid could neutralize the pancreatic enzymes that cause the increased deterioration of digestive function after surgery. Rault et al. [14] reported that PJ produced better pancreatic exocrine function than PG after PD. Lemaire et al. [15] also reported that most patients developed pancreas exocrine insufficiency after PD with PG. We expect that the PJt method could theoretically be more beneficial for preserving the exocrine function of the pancreas after surgery compared with the PGt method.
Second, the ease and simplicity of the PJt technique is a leading cause of changing anastomosis to PJt. PJt does not require suturing of the walls of tiny pancreatic ducts as in the conventional duct-to-mucosa method. Only two pancreas-penetrating sutures are required to complete the anastomosis without additional reinforcement sutures, and it is a quite small number of sutures compared with other previous techniques. Ohigashi et al. [16] also used straight needles with a penetrating suture in a U-like fashion; however, this procedure requires at least 4 to 8 pancreas sutures. The conveniences of our technique could help shorten the pancreaticoenteric anastomosis time and overall operation time. The shortening of the operation time could also be expected to reduce the physiologic stress on the patient, and it might ultimately help to speed up the recovery process.
Comparing the two similar techniques using transpancreatic sutures, PGt and PJt, the anastomosis time was shorter in PJt than in PGt. In PGt, the anterior gastrotomy as well as the posterior gastrotomy are needed to perform the anastomosis under direct vision, and the gastrotomy site has to be closed again. Moreover, the sufficient mobilization of the pancreas stump from the SMV or the retroperitoneum is a very important step in both PGt and PJt because the stability of the anastomosis can be achieved through the deep invagination of the pancreas into the stomach or jejunum. In the PGt anastomosis, the pancreas stump should be mobilized more upward for invaginating it securely into the posterior wall of the stomach, and this usually requires a longer length of pancreas mobilization than that needed with the PJt. The posterior wall of the stomach is not freely movable for displacement during the PG, and therefore, more sufficient mobilization of the pancreas stump without tension is needed despite the prolonged operation time. In contrast, the jejunum is more free to move, and it is more favorable for completing the anastomosis.
The incidence of grades B and C POPF in the groups using transpancreatic sutures (groups PGt and PJt) was zero, although these results were limited to hard pancreas cases. The results support that these procedures could be feasible and safe for preventing clinically significant POPFs after PD in hard pancreas cases. The authors propose that the characteristics of our procedures could be the reason for this benefit. Initially, instead of the conventional curved needle with a circumferential suture, a straight needle was used to minimize pancreatic laceration and parenchymal injury from needles or suture ligations. As described by Neychev and Saldinger [17], straightening the needle and using large needles with an increased radius would help to reduce the force required for passing the needle through the pancreas stump, and this could minimize the tissue deformation caused by the tangential shear stress of the needle. Second, 4 buttresses were introduced to minimize the gap between the jejunum wall and the pancreas, and this helped to make the anastomosis more secure. The buttresses could provide the required compressive force around the suture sites and reduce the space between adjacent stitches. We expect that the buttresses could also prevent the crushing of the pancreas tissue around the needle sites during tying. Furthermore, the buttresses could occlude the stitch holes on the anastomosis sites that were created by the needle. The potential gaps or the needle holes in the anastomosis could be one of the critical causes in the etiology of POPF as previously described by Torer et al. [18] and Peng et al. [19]. We propose that our use of buttresses could effectively help prevent POPF. Third, only two transpancreatic sutures were applied in our procedures, and the reduced number of sutures is expected to decrease the suture damage to the pancreas parenchyma and also reduce the risk of bleeding during the passage of the needle.
We have applied PJt anastomosis only for hard pancreas cases since 2013; however, the PGt anastomosis has also been selectively performed in patients with soft pancreases or the unusual condition in which there is a size mismatch between the pancreas and jejunum, such as in severe pancreatitis with edematous changes or with a bulky pancreas. In such cases, the enlarged pancreas stump is usually too hard to firmly invaginate into the lumen of the jejunum. Actually, in 2 patients, the method of anastomosis was changed to PGt from PJt during the operation. In this way, we selected the appropriate technique between PGt or PJt, and a tailored surgical approach could be employed based on the condition of the pancreas and viscera.
The results of this retrospective study should be interpreted with caution because of the study limitations. Selection bias could not be avoided, and there is concern that the type of anastomosis was chosen based on the surgeon's subjective judgment according to the clinical findings. To overcome these limitations, detailed indications and selection criteria for each anastomosis type should be accurately established. The current study also produced no data on the long-term outcomes related to pancreas exocrine insufficiency according to the type of anastomosis. The parameters related to digestive function should be evaluated in a future study to compare the exocrine function between the different types of anastomoses.
However, the PJt presented feasible and improved outcomes regarding POPF and the pancreaticoenteric anastomosis time despite these limitations. Moreover, this approach is also expected to be more easily applied in laparoscopic PD procedures. In previous reports using laparoscopic PD, the multiple sutures in the posterior pancreas wall has been the vulnerable point in the surgery due to the restrictions in suture motion, which is the one of fundamental limitations of laparoscopic surgery. However, in the case of PJt, only two transpancreatic sutures without additional reinforcements were sufficient to complete the anastomosis, which can reduce the operator burden even though it is only applicable in hard pancreas cases. The authors have performed 10 complete laparoscopic PDs successfully using this technique, although we did not describe those cases in this article.
In conclusion, we propose that this novel technique is very simple, easy, and safe for hard pancreas cases, and it is expected to have advantages over the conventional Du or PGt. We also expect that it could be useful for performing laparoscopic pancreaticoduodenectomies more comfortably. A well-designed randomized controlled prospective study with large sample sizes should be conducted in the near future to establish the superiority of this method over the conventional technique.
Figures and Tables
Fig. 1
A schematic diagram of pancreaticogastrostomy using two transpancreatic sutures with buttresses through a gastrostomy made in the anterior wall of stomach.
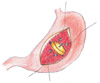
Fig. 2
The hardness of the pancreas has been estimated based on the surgeon's judgment during the operation and more objectively using a durometer to measure the hardness of the pancreas.
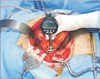
Fig. 3
Schematic diagrams of pancreaticogastrostomy using two transpancreatic sutures with buttresses (A) and pancreaticojejunostomy using two transpancreatic sutures with buttresses (B) after the completion of the reconstruction.
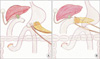
Fig. 4
Operative view (A, B) and schematic diagram (C) of pancreaticojejunostomy using two transpancreatic sutures with buttresses.

Table 1
Patient demographics and tumor characteristics
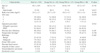
Values are presented as mean ± standard deviation or number (%).
Du, dunking method; PGt, pancreaticogastrostomy using transpancreatic sutures; PJt, pancreaticojejunostomy using transpancreatic sutures; ASA, American Society of Anesthesiologists; PPPD, pancreaticoduodenectomy; CBD, common bile duct.
a)PTBD (percutaneous transhepatic biliary drainage).
References
1. Belyaev O, Rosenkranz S, Munding J, Herzog T, Chromik AM, Tannapfel A, et al. Quantitative assessment and determinants of suture-holding capacity of human pancreas. J Surg Res. 2013; 184:807–812.
2. Hong TH, Youn YC, You YK, Kim DG. An easy and secure pancreaticogastrostomy after pancreaticoduodenectomy: transpancreatic suture with a buttress method through an anterior gastrotomy. J Korean Surg Soc. 2011; 81:332–338.
3. Foitzik T, Gock M, Schramm C, Prall F, Klar E. Octreotide hardens the pancreas. Langenbecks Arch Surg. 2006; 391:108–112.
4. Belyaev O, Herden H, Meier JJ, Muller CA, Seelig MH, Herzog T, et al. Assessment of pancreatic hardness-surgeon versus durometer. J Surg Res. 2010; 158:53–60.
5. Batignani G, Fratini G, Zuckermann M, Bianchini E, Tonelli F. Comparison of Wirsung-jejunal duct-to-mucosa and dunking technique for pancreatojejunostomy after pancreatoduodenectomy. Hepatobiliary Pancreat Dis Int. 2005; 4:450–455.
6. Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005; 138:8–13.
7. Walters DM, Stokes JB, Adams RB, Bauer TW. Use of a falciform ligament pedicle flap to decrease pancreatic fistula after distal pancreatectomy. Pancreas. 2011; 40:595–599.
8. Kim WS, Choi DW, Choi SH, Heo JS, Kim MJ, Song SC, et al. Clinical validation of the ISGPF classification and the risk factors of pancreatic fistula formation following duct-to-mucosa pancreaticojejunostomy by one surgeon at a single center. J Gastrointest Surg. 2011; 15:2187–2192.
9. Kuramoto M, Ikeshima S, Shimada S, Yamamoto K, Masuda T, Nakamura K, et al. Pancreaticojejunostomy by reinforcing the pancreas without covering the anastomotic line reduces pancreatic fistula. Int J Surg. 2013; 11:909–913.
10. Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007; 142:761–768.
11. Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007; 142:20–25.
12. Topal B, Fieuws S, Aerts R, Weerts J, Feryn T, Roeyen G, et al. Pancreaticojejunostomy versus pancreaticogastrostomy reconstruction after pancreaticoduodenectomy for pancreatic or periampullary tumours: a multicentre randomised trial. Lancet Oncol. 2013; 14:655–662.
13. Figueras J, Sabater L, Planellas P, Munoz-Forner E, Lopez-Ben S, Falgueras L, et al. Randomized clinical trial of pancreaticogastrostomy versus pancreaticojejunostomy on the rate and severity of pancreatic fistula after pancreaticoduodenectomy. Br J Surg. 2013; 100:1597–1605.
14. Rault A, SaCunha A, Klopfenstein D, Larroude D, Epoy FN, Collet D, et al. Pancreaticojejunal anastomosis is preferable to pancreaticogastrostomy after pancreaticoduodenectomy for longterm outcomes of pancreatic exocrine function. J Am Coll Surg. 2005; 201:239–244.
15. Lemaire E, O'Toole D, Sauvanet A, Hammel P, Belghiti J, Ruszniewski P. Functional and morphological changes in the pancreatic remnant following pancreaticoduodenectomy with pancreaticogastric anastomosis. Br J Surg. 2000; 87:434–438.
16. Ohigashi H, Ishikawa O, Eguchi H, Sasaki Y, Yamada T, Kishi K, et al. A simple and safe anastomosis in pancreaticogastrostomy using mattress sutures. Am J Surg. 2008; 196:130–134.
17. Neychev VK, Saldinger PF. Minimizing shear and compressive stress during pancreaticojejunostomy: rationale of a new technical modification. JAMA Surg. 2014; 149:203–207.
18. Torer N, Ezer A, Nursal TZ. Mattress sutures for the modification of end-to-end dunking pancreaticojejunostomy. Hepatobiliary Pancreat Dis Int. 2013; 12:556–558.
19. Peng SY, Wang JW, Lau WY, Cai XJ, Mou YP, Liu YB, et al. Conventional versus binding pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized trial. Ann Surg. 2007; 245:692–698.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download