Abstract
Purpose
To determine the prevalence of thrombophilia in Korean patients with an arterial thromboembolism (ATE) or a venous thromboembolism (VTE), and to evaluate the characteristic of VTE in patients with thrombophilia.
Methods
Hospital records of 294 patients (228 with VTE, 66 with ATE) including two foreign ones (mean age, 51.4 years) who underwent thrombophilia testing between August 2006 and March 2015 were reviewed retrospectively. In general, such screening was performed according to the guidelines of the international consensus statement for VTE. Thrombophilia testing included evaluations of the factor V Leiden and prothrombin G20210A mutations, levels of proteins C and S and antithrombin, and antiphospholipid antibody syndrome (APLS).
Results
A factor V Leiden mutation was not found in the 292 Korean patients. A prothrombin G21210A mutation was investigated in 33 patients but none was found. Among 226 Korean patients with VTE, 130 demonstrated no thrombophilia and 55 patients did after exclusion of 41 patients without confirmatory test. The most common form was protein S deficiency (31 of 55, 56%) followed by protein C deficiency, antithrombin deficiency, and APLS. When comparing patients with a VTE or deep vein thrombosis (DVT) according to the presence of thrombophilia, thrombophilia was associated with younger age (P = 0.001 for VTE; P < 0.001 for DVT) and a family history (P < 0.001 for VTE and DVT).
Thrombophilia is a condition that increases the risk of thromboembolic events via altered hemostasis, shifting the balance in favor of thrombus formation. This condition can be inherited or acquired. The most typical form of acquired thrombophilia is antiphospholipid antibody syndrome (APLS) and this can be detected as a lupus anticoagulant or as anticardiolipin antibodies. Inherited forms of thrombophilia include deficiencies in one of the three natural anticoagulants: antithrombin, proteins C and S, which have been linked to familial venous thrombosis. Inherited thrombophilia can also be caused by the factor V Leiden mutation, and by the prothrombin G20210A mutation, which predispose to dysfunctions in natural anticoagulants.
The mutation spectra of inherited thrombophilia in Asian patients differ from those in Western countries. Activated protein C (APC) resistance caused by a factor V Leiden mutation and an increased plasma prothrombin level caused by prothrombin G20210A mutation is known to be the most common cause of a venous thromboembolism (VTE) in Caucasian ethnic groups [1]. However, deficiencies in natural anticoagulants such as protein C, protein S, and antithrombin-III are known to be the most common causes of inherited thrombophilia in Asian patients [23].
In patients with a VTE and thrombophilia, the intensity and duration of anticoagulation therapy can help decrease the complications and mortality caused by a recurrent VTE; however, clinical evidence including randomized controlled trials is insufficient. Moreover, despite several studies on inherited thrombophilia in Western and in other Asian countries, there has been very little analysis on inherited thrombophilia in patients with a VTE or arterial thromboembolism (ATE) in Korean patients [45]. Therefore, the objective of this study was to determine the prevalence of thrombophilia in Korean patients with an ATE or VTE, and to evaluate the characteristics of the VTE in those with thrombophilia.
Between August 2006 and March 2015, 294 patients underwent screening for inherited thrombophilia due to an ATE or symptoms associated with a VTE at a single tertiary referral center, and these patients were eligible for this study. In general, such screening was performed for the patients according to the guidelines of the international consensus statement for VTE [1], and for patients with an ATE without a definite cause of thromboembolisms. Therefore, such screening for thrombophilia was not performed for all patients with an ATE or a VTE during the study period. The study was initiated after obtaining approval from the Institutional Review Board at our institution. The need for informed consent for the review of these medical records was not required by the board because this was a retrospective study.
All patients were admitted to hospital or presented to our outpatient clinic with symptoms associated with an ATE or a VTE. Screening for thrombophilia included evaluations of factor V Leiden and prothrombin G20210A mutations, measures of the levels of protein C (total antigen, activity) and protein S (total antigen, free form, activity), the antithrombin level, and the presence of APLS (lupus anticoagulant; IgG/M anticardiolipin antibodies) at the time of presentation. For evaluating inherited thrombophilia, tests for factor V Leiden and prothrombin G20210A mutations were performed at the initial presentation, and mutation was confirmed if the initial results were positive. Factor V Leiden and prothrombin G20210A mutations were evaluated using multiplex allele-specific polymerase chain reaction amplification. Protein C, protein S, and antithrombin levels were also tested at the time of initial presentation. However, these tests were usually repeated 2 weeks after the discontinuation of 3–6 months of anticoagulation therapy because their results are affected by acute-stage thrombosis and anticoagulant use in most patients. In our series, patients with low levels of these proteins at the initial presentation and in repeated test after the discontinuation of anticoagulants were defined as having deficiencies in these natural anticoagulants. For the diagnosis of APLS, the levels of IgG and IgM anticardiolipin antibodies, and lupus anticoagulant activity were tested at the time of presentation, and repeated at least 12 weeks apart. The diagnoses were referred to a rheumatologist for confirmation.
Outcomes of interest in our study were the prevalence of thrombophilia in Korean patients with an ATE or a VTE, and the characteristics of the VTE in patients with or without thrombophilia. For subgroup analysis in patients with a VTE and a deep vein thrombosis (DVT), patients were divided into 2 groups. Patients without thrombophilia (group I) were defined as having normal values for the abovementioned thrombophilia tests at the initial presentation or at repeated tests. Patients with thrombophilia (group II) were defined as having confirmed factor V Leiden or prothrombin mutations at the initial laboratory test, or with abnormally low levels of natural anticoagulants at repeated tests after temporary cessation of anticoagulation therapy, or those confirmed to have APLS. A provoked DVT was defined as one occurring in association with external risk factors, such as surgery, oral contraceptive use, trauma, immobility, or cancer; patients without such known factors were defined as having an unprovoked DVT.
Student independent t-tests were used to compare the differences of age in patients with and without inherited thrombophilia (i.e., groups I and II) because age follows normal distribution. Categorical variables were subjected to chi-square analysis if the sample size was adequate, or Fisher exact test if the sample size was small. All tests were two-sided and assumed significance at P < 0.05. Analyses were performed using IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA).
A total of 294 patients presented with symptoms associated with an ATE or VTE, including two foreign patients with a DVT. The mean patient age was 51.4 years (range, 11–86 years), and 189 (64.2%) were men. The underlying conditions used for screening of thrombophilia were associated with a VTE in 228 patients and with an ATE in 66. In 228 patients with a VTE, the mean age was 51.4 ± 17.1 years (range, 11–86 years) and 135 (59.2%) were men. The most common presenting disease was a DVT in a lower extremity in 152 patients (66.7%) followed by mesenteric venous thromboses in 35 (15.4%), superficial venous thromboses in 17 (7.5%), chronic venous insufficiency after a previous DVT in 17 (7.5%), and a DVT in an upper extremity in seven patients. In patients with an ATE, the mean age was 51.4 ± 16.8 years (range, 16–86 years), and 54 (82%) were men. The most common presenting disease was thromboembolism in a lower extremity in 43 patients (65%), followed by thromboembolism in an upper extremity in 17 (26%), and thromboembolism in the visceral arteries in six. The mean age was not significantly different between patients with VTE or ATE; however, male patients were more prevalent in those with an ATE (P = 0.001).
Factor V Leiden mutations were evaluated in all enrolled patients and the prothrombin G20210A mutation was tested in 33. One of the 2 foreign patients had a factor V Leiden mutation; however, this was not found in the 292 Korean patients. The prothrombin G20210A mutation was not found in any of the patients evaluated.
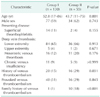
Natural anticoagulant deficiencies and APLS were also evaluated in all 292 Korean patients at the time of presentation. Among these, 117 demonstrated normal levels of natural anticoagulants and markers for APLS, and 175 revealed abnormally low levels of natural anticoagulants or positive results for APLS. Sixty patients did not receive repeated testing because they were lost to follow-up or for other reasons, and 115 patients underwent repeated testing for inherited thrombophilia. Among these patients, 59 demonstrated thrombophilia. Protein S deficiency was the most common deficiency and was found in 32 patients (54%) followed by protein C deficiency in 13 patients (22%), an antithrombin-III deficiency in 9 patients (15%), and APLS in 5 (Fig. 1).
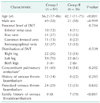
Among the 226 Korean patients with VTE, 41 did not receive repeated testing for inherited or acquired thrombophilia after initial abnormal results, so these patients were excluded. After exclusion, 130 patients were defined as group I and 55 patients including those with APLS were defined as group II (31 with protein S deficiency; 11 with protein C deficiency; 9 with antithrombin deficiency; and 4 with APLS; Fig. 1). Detailed characteristics of the 2 groups are given in Table 1. Among those with a VTE, patients in group II were significantly younger (P = 0.001) and had a significant family history (P < 0.001) compared with group I. Having had a previous VTE was more frequent in group II than in group I (P = 0.035).
Subgroup analysis was performed for Korean patients with a DVT in a lower extremity. Detailed characteristics of groups I and II are listed in Table 2. Patients in group II were significantly younger (P < 0.001) and had a significant family history (P < 0.001) compared with group I. However, having had a previous VTE was not significantly different between the groups.
Inherited thrombophilia is a major risk factor for having an idiopathic VTE and has a different prevalence between various ethnic groups [6]. In Western countries, APC resistance caused by factor V Leiden mutation is known to be the most common genetic predisposition, accounting for 20% of patients with a VTE [17]. However, this mutation is extremely rare in Asian patients with a VTE [28]. The main finding of the present study is that a factor V Leiden mutation was not found in 292 Korean patients with an ATE or a VTE. This is the largest series regarding this mutation in Korean patients with thromboembolisms to our knowledge. Importantly, the demographic features of our patients differed from the typical ones for patients with VTE. In general, the incidence of VTE rises exponentially with age and increases dramatically after the age of 60 years [9]. In addition, the proportion of patients with a provoked VTE ranges from 53% to 74% of first-time cases [910]. In our series, a provoked VTE only accounted for approximately 30% of the patients, meaning that many of our patients presented without specific risk factors for this syndrome. In addition, the mean age of patients with a VTE in our series was 51.4 years: younger than that reported for 'classic' cases [9]. Therefore, our patients with VTE can be considered as having had a high risk for inherited thrombophilia in consideration of their being younger and with a low incidence of provoked VTEs. However, we could not find any instance of a factor V Leiden mutation. Therefore, we suggest that testing for the factor V Leiden mutation in Korean patients with a VTE is unnecessary.
In contrast to the APC resistance caused by factor V Leiden or prothrombin mutations, natural anticoagulant deficiencies are known to be the main causes of inherited thrombophilia in Asian populations [2345]. Kim et al. [5] reported the frequency and mutation spectra of natural anticoagulant deficiencies in Korean patients with a VTE. In their series of 71 patients with a genetically confirmed natural anticoagulant deficiency, protein C deficiency was the most common (n = 36, 51%) followed by antithrombin deficiency (n = 21, 30%), and protein S deficiency (n = 14, 20%). In contrast to that study, reports based on Japanese and Chinese patients with a VTE revealed that protein S deficiency was the most common [21112]. In our series, protein S deficiency was also the most common deficiency (60%) followed by protein C and antithrombin deficiencies. Further large-scale studies are necessary to define the exact prevalence and frequency of natural anticoagulant deficiencies in the Korean population.
Some characteristics of patients with VTE are associated with thrombophilia. The most consistent finding is that it occurs in patients who are younger than those with a VTE without thrombophilia [71314]. In addition, a family history of thrombosis and spontaneous, recurrent, or life-threatening VTEs have been reported as the main clinical factors that increase the risk of thrombophilia [131516]. In our series, the characteristics of patients with a VTE and thrombophilia were similar to others reported. Being younger and having a significant family history were strongly associated with thrombophilia.
Several limitations of this study should be acknowledged. First, the major limitation was its retrospective nature, which could have been subject to selection bias. Testing for thrombophilia was provided to patients with suspected thrombophilia without definite risk factors for VTE, or to young patients. Therefore, features such as provoked thromboembolisms could have been affected by this selection bias. Second, many subjects were lost to follow-up for repeated testing for thrombophilia after a short-term discontinuation of anticoagulant therapy. This particular limitation prohibits us from assessing the exact prevalence and frequency of natural anticoagulant deficiency among our patients. Third, our definition of natural anticoagulant deficiency was not based on genetic sequencing, but was determined from abnormally low levels of natural anticoagulants in two test sessions. We tried to eliminate the effects of an acute stage and anticoagulants by repeated testing after short-term discontinuation of the therapy. However, coagulation test results are known to be affected by a variety of factors such as underlying diseases and medications, leading to false diagnoses of inherited deficiency [17]. Therefore, it is possible that we over- or underestimated the actual frequency of natural anticoagulant deficiency in our series.
In conclusion, we did not find any factor V Leiden mutation in Korean subjects with high risk of thrombophilia. Therefore, we suggest that such testing is unnecessary for Korean patients with a VTE. Protein S deficiency was the most common form of inherited thrombophilia in our series. Thrombophilia tended to be associated with a VTE in younger patients and family history of a VTE.
Figures and Tables
 | Fig. 1Flow chart of screening for thrombophilia in Korean patients with venous or arterial thromboembolisms. APLS, antiphospholipid antibody syndrome. |
References
1. European Genetics Foundation. Cardiovascular Disease Educational and Research Trust. International Union of Angiology. Mediterranean League on Thromboembolism. Nicolaides AN, Breddin HK, et al. Thrombophilia and venous thromboembolism. International consensus statement. Guidelines according to scientific evidence. Int Angiol. 2005; 24:1–26.
2. Shen MC, Lin JS, Tsay W. High prevalence of antithrombin III, protein C and protein S deficiency, but no factor V Leiden mutation in venous thrombophilic Chinese patients in Taiwan. Thromb Res. 1997; 87:377–385.
3. Zama T, Murata M, Ono F, Watanabe K, Watanabe R, Moriki T, et al. Low prevalence of activated protein C resistance and coagulation factor V Arg506 to Gln mutation among Japanese patients with various forms of thrombosis, and normal individuals. Int J Hematol. 1996; 65:71–78.
4. Kim TW, Kim WK, Lee JH, Kim SB, Kim SW, Suh C, et al. Low prevalence of activated protein C resistance and coagulation factor V Arg506 to Gln mutation among Korean patients with deep vein thrombosis. J Korean Med Sci. 1998; 13:587–590.
5. Kim HJ, Seo JY, Lee KO, Bang SH, Lee ST, Ki CS, et al. Distinct frequencies and mutation spectrums of genetic thrombophilia in Korea in comparison with other Asian countries both in patients with thromboembolism and in the general population. Haematologica. 2014; 99:561–569.
6. Ridker PM, Miletich JP, Hennekens CH, Buring JE. Ethnic distribution of factor V Leiden in 4047 men and women. Implications for venous thromboembolism screening. JAMA. 1997; 277:1305–1307.
7. Roldan V, Lecumberri R, Munoz-Torrero JF, Vicente V, Rocha E, Brenner B, et al. Thrombophilia testing in patients with venous thromboembolism. Findings from the RIETE registry. Thromb Res. 2009; 124:174–177.
8. Seki T, Okayama H, Kumagai T, Kumasaka N, Sakuma M, Isoyama S, et al. Arg506Gln mutation of the coagulation factor V gene not detected in Japanese pulmonary thromboembolism. Heart Vessels. 1998; 13:195–198.
9. White RH. The epidemiology of venous thromboembolism. Circulation. 2003; 107:23 Suppl 1. I4–I8.
10. Heit JA, O'Fallon WM, Petterson TM, Lohse CM, Silverstein MD, Mohr DN, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med. 2002; 162:1245–1248.
11. Hamasaki N, Kuma H, Tsuda H. Activated protein C anticoagulant system dysfunction and thrombophilia in Asia. Ann Lab Med. 2013; 33:8–13.
12. Kinoshita S, Iida H, Inoue S, Watanabe K, Kurihara M, Wada Y, et al. Protein S and protein C gene mutations in Japanese deep vein thrombosis patients. Clin Biochem. 2005; 38:908–915.
13. Mateo J, Oliver A, Borrell M, Sala N, Fontcuberta J. Laboratory evaluation and clinical characteristics of 2,132 consecutive unselected patients with venous thromboembolism: results of the Spanish Multicentric Study on Thrombophilia (EMET-Study). Thromb Haemost. 1997; 77:444–451.
14. Seligsohn U, Lubetsky A. Genetic susceptibility to venous thrombosis. N Engl J Med. 2001; 344:1222–1231.
15. Weingarz L, Schwonberg J, Schindewolf M, Hecking C, Wolf Z, Erbe M, et al. Prevalence of thrombophilia according to age at the first manifestation of venous thromboembolism: results from the MAISTHRO registry. Br J Haematol. 2013; 163:655–665.
16. Di Minno MN, Ambrosino P, Ageno W, Rosendaal F, Di Minno G, Dentali F. Natural anticoagulants deficiency and the risk of venous thromboembolism: a meta-analysis of observational studies. Thromb Res. 2015; 135:923–932.
17. De Stefano V, Rossi E, Paciaroni K, Leone G. Screening for inherited thrombophilia: indications and therapeutic implications. Haematologica. 2002; 87:1095–1108.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download