Abstract
IgG4-related disease is a relatively new disease entity characterized by elevated serum IgG4 levels and marked infiltration of IgG4-positive plasma cells in lesions. Organ enlargement or nodular lesions consisting of abundant infiltration of lymphocytes and IgG4-positive plasma cells and fibrosis are seen in various organs throughout. We encountered a patient with an inflammatory pseudotumor of the rectum, which was histopathologically confirmed to be an IgG4-related disease. The patient was a 28-year-old woman who had constipation for 3 months. The endoluminal ultrasonography showed a lesion that was heterogeneous and low echogenic in lower rectum. The result of colonoscopic biopsy findings was of chronic proctitis with lymphoid aggregates. For a confirmative diagnosis, excision was performed. Histopathological examination represented plasma cell infiltration and fibrosis. Immunohistochemistry revealed prominence of IgG4-positive plasma cells and confirmed the diagnosis of IgG4-related disease. The patient is currently under observation on low-dose oral prednisolone without relapse.
IgG4-related disease (RD) is a recently recognized systemic condition characterized by elevated serum IgG4 levels and responsiveness to steroids. IgG4-RD shows organ enlargement or nodular lesions consisting of abundant infiltration of lymphocytes and IgG4-positive plasma cells and fibrosis. IgG4-RD affects various internal organs such as pancreas, bile duct, gallbladder, liver, salivary gland, lacrimal gland, retroperitoneum, and lymph nodes metachronously [12]. IgG4-RD frequent presents with clinical and radiological findings that mimic a malignancy, resulting in unnecessary resection, according to comprehensive clinical diagnostic criteria for IgG4-RD [3]. IgG4-RD is diagnosed when there is a characteristically diffuse or localized swelling in single or multiple organs with elevated serum IgG4 levels, or when there are histological findings of abundant infiltration of IgG4-positive plasma cells and lymphocytes, along with fibrosis.
IgG4-RD shows older male predominance, with most patients in their 60's [4]. IgG4-RD in the low rectum is extremely rare and this may be the first case report among the literature review. In this study, we report a patient with IgG4-RD of the low rectum.
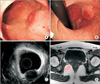
In February 2014, a 28-year-old Korean woman presented herself to the Department of Surgery at Seoul St. Mary's Hospital, with a lower rectal mass. She had previously been in good health. She had a slight traumatic injury in her 3rd right finger and then post conservative therapy. However, the radiating pain of her forearm was prolonged so she consulted an anesthesiologist. Her pain was intermittent. Incidentally, she had a colonoscopy due to repeated constipation and anal discomfort. A 2-cm-sized ovoid protruding mass was found 2 cm above anal verge at the anterior rectal wall (Fig. 1A, B). Accordingly, the digital rectal examination revealed a firm mass 2 cm above the anal verge. The colonoscopic endoluminal ultrasonography also revealed a 2-cm-sized heterogeneous and low echogenic lesion involving mucosal, submucosal, and muscularis propria layer (Fig. 1C). The initial colonoscopic histopathological examination confirmed chronic proctitis with lymphoid aggregates and atrophy. The laboratory data were as follows (numbers in parentheses indicate the normal range of values): white blood cell count, 7,390/mm3 (4,000 to 10,000/mm3); hemoglobin, 13.1 g/dL (12 to 16 g/dL); hematocrit, 38.7% (34% to 49%); platelet count, 329,000/mm3 (150,000 to 450,000/mm3); aspartate aminotransferase, 17 IU/L (14 to 40 IU/L); alanine aminotransferase, 17 IU/L (9 to 45 IU/L); alkaline phosphatase, 48 IU/L (30 to 120 IU/L); total bilirubin, 0.9 mg/dL (0.47 to 1.58 mg/dL); amylase, 122 U/L (48 to 176 U/L); total protein, 7.1 g/dL (6.6 to 8.3 g/dL); albumin, 4.7 g/dL (3.5 to 5.2 g/dL); HBsAg negative, hepatitis B surface antibody positive. The serum levels of carcinoembryonic antigen and α-FP were within normal limits. The CT revealed a 2.1-cm-sized protruding mass, which was slightly enhanced on the right anterior wall of the lower rectum. Accordingly, the MRI revealed a mass about 1.4 cm under T1 and T2 low signal intensity, abutting the right anterior wall of the lower rectum. T2 signal intensities are not typical for gastrointestinal stromal tumors (Fig. 1D). However, this lesion showed bright homogeneous enhancement patterns. There was no definite evidence of lymphadenopathy around the rectum. Our impression was a submucosal tumor involving the anterior wall of the right lower rectum, such as gastrointestinal stromal tumor, leiomyoma, or neuroendocrine tumor. For a differential diagnosis, the patient underwent an open excision biopsy by transanal approach. The pathological examination of the frozen specimen obtained during the operation helped reveal an atypical lympho-proliferative type, and no malignant component.
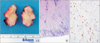
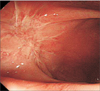
The lesion lies on the anterior wall of rectum. The mass is 2 cm × 3 cm sized and it is firm (Fig. 2A). The histological examination revealed a sclerosing nodular lesion with diffuse submucosal fibrosis and sclerosis (Fig. 2B). The immunohistochemical staining for IgG4 was prominent among the plasma cells (>50/high power field) and the ratio of IgG4-positive to IgG-positive plasma cells was very high (Fig. 2C). Accordingly, the patient was diagnosed as having IgG4-RD of the low rectum and we consulted the rheumatology department. The patient was treated with prednisolone at the dosage of 40 mg/day for 14 days, followed by 28 mg/day for 14 days, and 20 mg/day for another 14 days. She has subsequently been maintained on 10 mg/day of prednisolone. After 1 month of prednisolone therapy, she noted improvement of anal discomfort and right forearm radiating pain. The follow-up examinations and colonoscopy have shown no other discomfort and intact anastomosis.
In conclusion, our case demonstrates that IgG4-RD is difficult to diagnose preoperatively and requires steroid therapy. IgG4-RD in the low rectum is an extremely rare case and may be the first case report among the literature review. In this study, we report a patient with IgG4-RD of the low rectum (Fig. 3).
Since the introduction of IgG4-RD, the characteristics of the disease have evolved continuously. IgG4-RD is a systemic disease characterized by extensive IgG4-positive plasma cells and T-lymphocyte infiltration of various internal organs. It was first introduced by Hamano et al. [56], who reported an autoimmune pancreatitis (AIP) patient with characteristically elevated serum IgG4 levels, and IgG4-positive infiltrating plasma cells. It is known that AIP is often associated with extrapancreatic clinical manifestations, including sclerosing cholangitis, peritoneal and mediastinal fibrosis, and inflammatory pseudotumor of lung, liver, thymus or interstitial nephritis [17]. A report emphasizes the role of T cells in pathophysiology of the disease. Naive T cells differentiate into helper effector T cells. IgG4-RD is characterized by a Th2 response and increased expression of Th2 associated cytokines (IL-4, 5, 10, 13). Regulatory cytokines such as IL-10 and TGF-beta might also be closely involved in the pathogenesis of IgG4-RD. IL-10 switches IgG4 production, and TGF-beta is a powerful fibrogenic cytokine [8]. Most IgG4-related systemic disease has been found to be associated with AIP but those without pancreatic involvement have also been reported [9].
Treatment of IgG4-RD includes administration of corticosteroids and surgical resection [10]. Especially, the disease is considered as a chronic inflammatory disease, and steroid therapy is favored due to its well-known anti-inflammatory effects. However, biopsy of rectal masses should be performed for differential diagnosis. In this case, we performed a transanal excision in consideration of her improved quality of life, which was seriously deteriorated due to the mass’s effect; she had suffered from intermittent constipation and defecation difficulty. It was inevitable that the unconfirmed mass be resected.
Figures and Tables
Fig. 1
Findings of imaging studies of IgG4-related disease of rectum. (A, B) Colonoscopic view of low rectum: 2-cm-sized protruding mass; (C) colonoscopic endoluminal ultrasonography view: 2-cm-sized heterogeneous low echogenic lesion involving mucosal, submucosal and proper muscle layer; (D) T1-weighted MRI. A 1.4-cm T1 low signal intensity mass (arrow) involving or abutting right anterior wall of lower rectum.
ACKNOWLEDGEMENT
The authors appreciate NaYeon Kang (Inglemoor high school, WA, USA) for her contribution to this paper in editing English and photography.
References
1. Kamisawa T, Funata N, Hayashi Y, Eishi Y, Koike M, Tsuruta K, et al. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003; 38:982–984.
2. Kamisawa T, Takuma K, Egawa N, Tsuruta K, Sasaki T. Autoimmune pancreatitis and IgG4-related sclerosing disease. Nat Rev Gastroenterol Hepatol. 2010; 7:401–409.
3. Research Committee to establish diagnostic criteria and development of treatment for systemic IgG4-related sclerosing disease. Research Committee to establish a new clinical entity, IgG4-related multiorgan lymphoproliferative syndrome (IgG4-MOLPS). Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD),2011. Research Program of Intractable Disease provided by the Ministry of Health, Labor, and Welfare of Japan. Nihon Naika Gakkai Zasshi. 2012; 101:795–804.
4. Saeki T, Ito K, Yamazaki H, Imai N, Nishi S. Hypocomplementemia of unknown etiology: an opportunity to find cases of IgG4-positive multi-organ lymphoproliferative syndrome. Rheumatol Int. 2009; 30:99–103.
5. Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001; 344:732–738.
6. Hamano H, Kawa S, Ochi Y, Unno H, Shiba N, Wajiki M, et al. Hydronephrosis associated with retroperitoneal fibrosis and sclerosing pancreatitis. Lancet. 2002; 359:1403–1404.
7. Hamano H, Arakura N, Muraki T, Ozaki Y, Kiyosawa K, Kawa S. Prevalence and distribution of extrapancreatic lesions complicating autoimmune pancreatitis. J Gastroenterol. 2006; 41:1197–1205.
8. Zen Y, Fujii T, Harada K, Kawano M, Yamada K, Takahira M, et al. Th2 and regulatory immune reactions are increased in immunoglobin G4-related sclerosing pancreatitis and cholangitis. Hepatology. 2007; 45:1538–1546.
9. Kamisawa T, Okamoto A. IgG4-related sclerosing disease. World J Gastroenterol. 2008; 14:3948–3955.
10. Maruya S, Miura K, Tada Y, Masubuchi T, Nakamura N, Fushimi C, et al. Inflammatory pseudotumor of the parapharyngeal space: a case report. Auris Nasus Larynx. 2010; 37:397–400.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download