Abstract
Purpose
Depth of wall invasion is an important prognostic factor in patients with gastric cancer, whereas the prognostic significance of intraoperative macroscopic serosal invasion (mSE) findings remain unclear when they show a discrepancy in pathologic findings. This study, therefore, assessed the prognostic significance of mSE.
Methods
Data from cohort of 2,835 patients with resectable gastric cancer who underwent surgery between 1990 and 2010 were retrospectively reviewed.
Results
The overall accuracy of mSE and pathologic results was 83.4%. The accuracy of mSE was 75.5% in pT2. On the other hand, the accuracy of pT3 dropped to 24.5%. According to mSE findings (+/–), the 5-year disease-specific survival (DSS) rate differed significantly in patients with pT2 (+; 74.2% vs. –; 92.0%), pT3 (+; 76.7% vs. –; 91.8%) and pT4a (+; 51.3% vs. –; 72.8%) (P < 0.001 each), but not in patients with T1 tumor. Multivariate analysis showed that mSE findings (hazard ratio [HR], 2.275; 95% confidence interval [CI], 1.148–4.509), tumor depth (HR, 6.894; 95% CI, 2.325–20.437), nodal status (HR, 5.206; 95% CI, 2.298–11.791), distant metastasis (HR, 2.881; 95% CI, 1.388–6.209), radical resection (HR, 2.002; 95% CI, 1.017–3.940), and lymphatic invasion (HR, 2.713; 95% CI, 1.424–5.167) were independent predictors of 5-year DSS rate.
Conclusion
We observed considerable discrepancies between macroscopic and pathologic diagnosis of serosal invasion. However, macroscopic diagnosis of serosal invasion was independently prognostic of 5-year DSS. It suggests that because the pathologic results could not be perfect and the local inflammatory change with mSE(+) could affect survival, a combination of mSE(+/–) and pathologic depth may be predictive of prognosis in patients with gastric cancer.
Early detection, rational lymphadenectomy, and the development of several therapeutic modalities have improved the survival of patients with gastric cancer [123]. Nevertheless, gastric cancer remains the fourth most common malignant tumor and the second leading cause of cancer-related deaths worldwide [4]. The most important prognostic indicators in gastric cancer are depth of wall invasion (pT) and the status of lymph node metastasis (pN) [567]. Therefore, accurate determination of invasive depth and lymph node metastasis, or the optimization of pT and pN categories, is critical for determining the extent of disease, guiding treatment planning, and predicting outcomes [8].
Peritoneal recurrence is common after curative resection of serosa positive gastric cancer because of free intraperitoneal cancer cells exfoliated from the serosal surface [9]. Although histologically determined pathologic serosal invasion is an important prognostic factor in patients with gastric cancer [10], the prognostic significance of intraoperative macroscopic serosal invasion (mSE) remains unclear. Discrepancies between macroscopic and pathologic serosal invasion have been reported [111213]. To date, however, few studies have evaluated the clinical significance of mSE. This study, therefore, assessed the prognostic significance of mSE.
Cohort data of the 3,571 patients who underwent surgery for primary gastric adenocarcinoma from January 1990 to December 2010 at the Department of Surgery, Chonbuk National University Medical School, were retrospectively reviewed. Patients with macroscopic organ invasion, patients who had received neo- adjuvant chemotherapy, patients who had undergone exploratory laparotomy or bypass surgery, and patients lacking a description of intraoperative serosal findings were excluded. The remaining 2,835 patients were included in the present study.
Clinicopathologic features were evaluated, including patient age, gender, extent of resection, tumor size, Borrman type, tumor differentiation, tumor depth and lymph nodal status. Lymph node metastasis and depth of tumor invasion were classified according to the 7th edition of the American Joint Committee on Cancer/Union for International Cancer Control TNM staging system [14]. In patients with multiple synchronous gastric cancers, the lesion with the deepest infiltration of the gastric wall was regarded as the main lesion and any others were regarded as accessory lesions. The clinicopathological characteristics of the main lesion were used for the analysis. Extent of lymph node dissection was determined using the recommendations of the Japanese Research Society for Gastric Carcinoma [15]. Resections were deemed curative when no gross residual disease was evident at the time of operation, with tumor-free resection margins on histological examination. During surgery, patients with any evidence of hard and nodular texture and/or color change of the serosal surface of the primary tumor were estimated as having mSE(+) by operator.
Not all patients were given postoperative chemotherapy. Patients with advanced gastric cancer above TNM stage II were given 5-fluorouracil-based postoperative chemotherapy, starting within 3 weeks after surgery. No patients were given postoperative or preoperative radiotherapy.
In general, follow-up consisted of abdomino-pelvic computed tomogram every 6 months for 5 years after surgery and esophago-gastro-duodenoscopy annually for 5 years after surgery. Cancer recurrence was defined as positive radiological evidence. Follow-up of patients was completed until the cutoff date of December 31, 2013. At the time of the last follow-up, 334 patients (11.8%) had been lost to follow up. The median follow-up interval at the cutoff date was 50 months (range, 0–232 months).
All statistical analyses were performed using the SPSS ver. 15.0 (SPSS, Inc., Chicago, IL, USA). Categorical variables were compared by chi-square tests and continuous variables by two-tailed t-tests. Disease-specific survival (DSS) rates were determined using the Kaplan-Meier method and compared using log-rank tests. Five-year DSS rates were estimated according to T category and compared for each T category between mSE(+) and mSE(–) patients. Multivariable Cox regression was used to identify factors significantly correlated with prognosis. For all analyses, P values <0.05 were considered statistically significant.
All information was obtained according to the Chonbuk National University Hospital Institutional Review Board and data were collected without revealing any personal information (IRB No. 2015-02-014).
Of the total of 2,835 patients, 1,946 (68.6%) were male patients and 889 (31.4%) were female, with a male to female ratio of 2.19:1. Mean patient age was 59.0 (standard deviation, 11) years. Of the 2,835 patients, 2,453 (86.5%) underwent partial gastrectomy and 382 (13.5%) underwent total gastrectomy. Pathologic examination classified 1,590 patients (56.8%) as pT1, 368 (12.9%) as pT2, 396 (13.9%) as pT3, and 481 (16.9%) as pT4a. Lymph node metastasis was found in 979 patients (34.5%) (Table 1).
Fig. 1 shows a relationship of mSE(+/–) and pSE(+/–). mSE(–) was in 62 of the 481 pSE(+) patients (12.9%), whereas mSE(+) was in 444 of the 2,354 pSE(-) patients (18.9%). The overall accuracy of mSE(+/–) was 82.1%, its sensitivity and specificity were 87.1% and 81.1%, respectively, and its positive and negative predictive values were 48.6% and 96.9%, respectively. Rates of over-estimation into mSE(+) in patients with pT1, pT2, and pT3 were 3.5% (55 of 1,590), 24.5% (90 of 368), 75.5% (299 of 396), respectively. pT4a was underestimated to mSE(–) by 12.9%.
The 5-year DSS in patients with pT1, pT2, pT3, and pT4a were 98.7%, 88.1%, 80.7%, and 54.1%, respectively (Fig. 2). Although 5-year DSS were similar in patients with mSE(+) and mSE(–) in pT1 tumors, they were significantly higher in patients with mSE(–) than mSE(+) tumors classified as stages pT2 (92.0% vs. 74.2%, P < 0.001), pT3 (91.8% vs. 76.7%, P < 0.001), and pT4a (72.8% vs. 51.3%, P < 0.001) (Fig. 3).
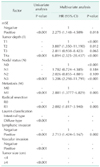
Table 2 shows the Cox regression analysis of factors significantly associated with 5-year DSS. Univariate analysis showed that mSE, tumor depth, nodal status, distant metastasis, radical resection, Lauren classification, lymphatic invasion, vascular invasion, and tumor size were significantly associated with 5-year DSS. Multivariate analysis showed that mSE (HR, 2.275; 95% CI, 1.148–4.509), tumor depth (HR, 6.894; 95% CI, 2.325–20.437), nodal status (HR, 5.206; 95% CI, 2.298–11.791), distant metastasis (HR, 2.881; 95% CI, 1.388–6.209), radical resection (HR, 2.002; 95% CI, 1.017–3.940), and lymphatic invasion (HR, 2.713; 95% CI, 1.424–5.167) were independent predictors of 5-year DSS.
In patients with pSE(+) who received curative gastrectomy, as high as 50% of the patients died from peritoneal recurrence within 2 years. Peritoneal recurrence is the most common form of gastric cancer recurrence. [191617] Therefore, pSE(+/–) is one of the most important factors in predicting prognosis, also it is important in controlling local recurrence and in choosing postsurgery treatment strategies [18]. Also, the finding of mSE(+/–) is important for determining in-surgery strategy as the operator has no indications of pSE during surgery.
Jeong et al. [13] reported mSE accuracy as 88%, sensitivity 82%, and specificity 89%. This is similar to our result, which showed mSE accuracy as 82%, sensitivity 87.1%, and specificity 81.1%. However, in analyzing pT category (Fig. 1B), the accuracy in pT2 was 75.5%, it in pT3 was 24.5% showing a marked decrease in accuracy. Kim et al. [19] reported pT3 accuracy as 46.4% and Jeong et al. [13] reported the sum accuracy of pT2 and pT3 as 65.3%. This means that there is a greater chance to overestimate pSE(–) into mSE(+) being the determining factor for in-surgery strategy.
In terms of accuracy of pSE(+/–), it is difficult to describe because pathologic results are the final determination of tumor depth. If we consider pathologic results as an indicator of absolutely accurate real tumor depth, there should have been no difference in survival within mSE(+/–) of pT group subdivisions. However, there was a difference in survival. While this result raises the question as to whether pSE(+/–) results can be trusted, other reports have also shown that there is a problem in completely trusting the results of pSE(+/–) [1920]. Kim et al. [19] and Wang et al. [20] showed similar survival in pT3 and pT4 patients with mSE(+). In our results, pT2 and 3 patients with mSE(+) had similar survival with pT4a with mSE(–). These results show the clinical importance of mSE and raises questions regarding pSE.
Macroscopic judgement of serosal involvement depends on the operator's subjective decisions that are based on the serosal change of color and/or texture comparative to the neighboring normal serosal surface. Serosal change without cancer penetration might be related to inflammation, which plays a role in all stages of tumorigenesis. Inflammatory microenvironment surrounding tumor cells promotes tumor growth, angiogenesis, and metastasis [21]. Furthermore, systemic inflammatory responses have a role in survival prediction in patients with primary operable cancer [22]. While C-reactive protein level, neutrophil, and platelet counts represent the systemic inflammatory reaction, we postulate that macroscopic serosal change might reflect local inflammatory reaction which affects survival rates, except T1. We suppose that T1 is too small to affect survival despite the serosal change.
The microscopic view of serosal involvement demands meticulous pathologic analysis and may require extensive sampling and/or serial sectioning. Thus, it can be missed on routine histopathologic examination. In addition, the histopathologic findings associated with peritoneal penetration are heterogeneous, and standard guidelines for their diagnostic interpretation are lacking. Since there are apparent inconsistencies in the histological definitions of peritoneal invasion, these problems result in both substantial interobserver variation and underdiagnosis of peritoneal involvement because most pathologists tend to err on the side of conservative interpretation [23].
Although depth of tumor invasion within the stomach wall is reflected in TNM stage, the area of invasion is not. Close relationships have been reported between the rate of detection of intraperitoneal free cancer cells and the area of serosal invasion, and between the latter and prognosis of patients who have gastric cancer with serosal invasion [1724]. While this study does not show that difference in survival based on the surface area, the results showing a higher survival in T4a with mSE(–) (5-year DSS; 72.8%) compared to T4a with mSE(+) (5-year DSS; 51.3%) could be based on the smaller surface area of invasion. Cases where the operator determined T4a tumor as mSE(–) during surgery could be because the involved serosa did not have a significant change in characteristic and small size. If we consider serosal invasion with this characteristic as minor pSE(+), the T2&3 with mSE(+) that showed a similar survival with T4a with mSE(–) could be considered minor pSE(+).
Diagnostic modalities that may improve the macroscopic diagnosis of serosal invasion include intraoperative and endoscopic ultrasonographic determination of tumor depth [25]. The presence of peritoneal free cancer cells derived from the serosa may be determined by analyzing peritoneal lavage fluid. For example, tumor markers in peritoneal lavage fluid may be predictive of peritoneal carcinomatosis [26]. Molecular methods that are more sensitive and more rapid than conventional peritoneal lavage fluid cytology can be used to detect peritoneal free cells [10]. Further studies are required to improve the accuracy of macroscopic serosal findings and to detect peritoneal free cancer cells.
This study had several limitations, including its retrospective design and inclusion of patients treated at a single center. Determination of intraoperative macroscopic serosal invasion epended primarily on subjective measurements by surgeons. Since many surgeons were included in this study, bias may have been introduced. Prospective, multicenter, large-scale studies analyzing the prognostic significance of macroscopic serosal invasion are required to confirm our results.
In conclusion, we observed considerable discrepancies bet ween macroscopic and pathologic diagnosis of serosal invasion. However, macroscopic diagnosis of serosal invasion was independently prognostic of 5-year DSS. It suggests that because the pathologic results could not be perfect and local inflammatory change with mSE(+) could affect the survival, a combination of mSE(+/–) and pathologic tumor depth may be predictive of prognosis in patients with gastric cancer.
Figures and Tables
Fig. 1
Macroscopic serosal invasion (mSE) and pathologic serosal invasion (pSE) show a discrepancy. (A) Overestimation into mSE(+) among pSE(–;) is 18.9%. Underestimation into mSE(–;) among pSE(+) is 12.9%. We could calculate sensitivity (87.1%), specificity (81.1%), positive predictive value (48.6%), negative predictive value (96.9%) and accuracy (82.1%) of mSE. (B) It shows the discrepancy according to pT category. pT1 (3.5%), pT2 (24.5%) and pT3 (75.5%) were overestimated to mSE(+). T4a (12.9%) was underestimated to mSE(–;).

Fig. 2
Five-year disease-specific survival rate according to tumor depth (pT category). Five-year disease-specific survival rate of pT1, pT2, pT3, and pT4a were 98.7%, 88.1%, 80.7%, and 54.1%, respectively.

Fig. 3
Five-year disease-specific survival rate in pT1.T4a according to mSE(+/–). (A) Five-year disease-specific survival rates were similar in patients with mSE(+) and mSE(–;) of T1 tumors. (B-D) Five-year disease-specific survival rates were significantly higher in patients with mSE(–;) than mSE(+) tumors of pT2 (92.0% vs. 74.2%, P < 0.001), pT3 (91.8% vs. 76.7%, P < 0.001), and pT4a (72.8% vs. 51.3%, P < 0.001). a)Macroscopic finding of serosal invasion. b)Indicates 5-year disease-specific survival rate. mSE, macroscopic serosal invasion.

ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0005274).
References
1. Takagane A, Terashima M, Abe K, Araya M, Irinoda T, Yonezawa H, et al. Evaluation of the ratio of lymph node metastasis as a prognostic factor in patients with gastric cancer. Gastric Cancer. 1999; 2:122–128.
2. Lindsey H. Preoperative chemoradiotherapy shows promise in gastric cancer. Lancet Oncol. 2004; 5:519–519.
3. Shimoyama S, Seto Y, Yasuda H, Mafune H, Kaminishi M. Concepts, rationale, and current outcomes of less invasive surgical strategies for early gastric cancer: data from a quarter-century of experience in a single institution. World J Surg. 2005; 59:58–65.
4. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009; 59:225–249.
5. Park do J, Kong SH, Lee HJ, Kim WH, Yang HK, Lee KU, et al. Subclassification of pT2 gastric adenocarcinoma according to depth of invasion (pT2a vs pT2b) and lymph node status (pN). Surgery. 2007; 141:757–763.
6. Lee CC, Wu CW, Lo SS, Chen JH, Li AF, Hsieh MC, et al. Survival predictors in patients with node-negative gastric carcinoma. J Gastroenterol Hepatol. 2007; 22:1014–1018.
7. Kunisaki C, Shimada H, Nomura M, Matsuda G, Otsuka Y, Ono H, et al. Clinical impact of metastatic lymph node ratioin advanced gastric cancer. Anticancer Res. 2005; 25(2B):1369–1375.
8. Wang W, Sun XW, Li CF, Lv L, Li YF, Chen YB, et al. Comparison of the 6th and 7th editions of the UICC TNM staging system for gastric cancer: results of a Chinese single-institution study of 1,503 patients. Ann Surg Oncol. 2011; 18:1060–1067.
9. Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000; 87:236–242.
10. Fujiwara Y, Okada K, Hanada H, Tamura S, Kimura Y, Fujita J, et al. The clinical importance of a transcription reverse-transcription concerted (TRC) diagnosis using peritoneal lavage fluids in gastric cancer with clinical serosal invasion: a prospective, multicenter study. Surgery. 2014; 155:417–423.
11. Yasuda K, Shiraishi N, Inomata M, Shiroshita H, Izumi K, Kitano S. Prognostic significance of macroscopic serosal invasion in advanced gastric cancer. Hepatogastroenterology. 2007; 54:2028–2031.
12. Ichiyoshi Y, Maehara Y, Tomisaki S, Oiwa H, Sakaguchi Y, Ohno S, et al. Macroscopic intraoperative diagnosis of serosal invasion and clinical outcome of gastric cancer: risk of underestimation. J Surg Oncol. 1995; 59:255–260.
13. Jeong O, Ryu SY, Jeong MR, Sun JW, Park YK. Accuracy of macroscopic intraoperative diagnosis of serosal invasion and risk of over- and underestimation in gastric carcinoma. World J Surg. 2011; 35:2252–2258.
14. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17:1471–1474.
15. Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition -. Gastric Cancer. 1988; 1:10–24.
16. Ribeiro U Jr, Gama-Rodrigues JJ, Safatle-Ribeiro AV, Bitelman B, Ibrahim RE, Ferreira MB, et al. Prognostic significance of intraperitoneal free cancer cells obtained by laparoscopic peritoneal lavage in patients with gastric cancer. J Gastrointest Surg. 1998; 2:244–249.
17. Ikeguchi M, Oka A, Tsujitani S, Maeta M, Kaibara N. Relationship between area of serosal invasion and intraperitoneal free cancer cells in patients with gastric cancer. Anticancer Res. 1994; 14(5B):2131–2134.
18. Sheen-Chen SM, Chou CW, Chen MC, Chen FC, Chen YS, Chen JJ. Adenocarcinoma in the middle third of the stomach: an evaluation for the prognostic significance of clinicopathological features. Hepatogastroenterology. 1997; 44:1488–1494.
19. Kim DJ, Lee JH, Kim W. Impact of intra-operative macroscopic diagnosis of serosal invasion in pathological subserosal (pT3) gastric cancer. J Gastric Cancer. 2014; 14:252–258.
20. Wang HH, Huang JY, Wang ZN, Sun Z, Li K, Xu HM. Macroscopic serosal classification as a prognostic index in radically resected stage pT3-pT4b gastric cancer. Ann Surg Oncol. 2016; 23:149–155.
21. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010; 140:883–899.
22. Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010; 6:149–163.
23. Shepherd NA, Baxter KJ, Love SB. The prognostic importance of peritoneal involvement in colonic cancer: a prospective evaluation. Gastroenterology. 1997; 112:1096–1102.
24. Kaibara N, Iitsuka Y, Kimura A, Kobayashi Y, Hirooka Y, Nishidoi H, et al. Relationship between area of serosal invasion and prognosis in patients with gastric carcinoma. Cancer. 1987; 60:136–139.
25. Bentrem D, Gerdes H, Tang L, Brennan M, Coit D. Clinical correlation of endoscopic ultrasonography with pathologic stage and outcome in patients undergoing curative resection for gastric cancer. Ann Surg Oncol. 2007; 14:1853–1859.
26. Yamamoto M, Yoshinaga K, Matsuyama A, Tsutsui S, Ishida T. CEA/CA72-4 levels in peritoneal lavage fluid are predictive factors in patients with gastric carcinoma. J Cancer Res Clin Oncol. 2014; 140:607–612.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download