Abstract
Sclerosing encapsulating peritonitis (SEP) is a rare cause of intestinal obstruction that is characterized by a thick fibrotic membrane encasing the small intestine like a cocoon. Accurate preoperative diagnosis is often difficult. We present 2 cases of SEP that were diagnosed preoperatively by contrast-enhanced computed tomography scan. A 38-year-old man and a 56-year-old woman were admitted to Daegu Catholic University Medical Center because of recurrent intestinal obstruction. We performed exploratory laparotomy with doubt of the preoperative diagnosis of SEP. We confirmed the diagnosis of SEP on laparotomy and performed adhesiolysis. Both patients recovered successfully and had no signs of recurrence. A better awareness of SEP and its radiological features should lead to more correct preoperative diagnosis and result in more appropriate management, including surgery.
Sclerosing encapsulating peritonitis (SEP) is a rare cause of intestinal obstruction, which is characterized by a fibro-collagenous membrane enveloping the small intestine like a cocoon [123]. This disease can be classified as primary (idiopathic) or secondary according to the etiology [23]. The primary form, also known as abdominal cocoon syndrome, was first described by Foo et al. [1] in 1978. The pathognomonic feature of SEP is an encapsulating thick, whitish peritoneum, partially or totally encasing the small intestine, that can extend to involve other intra-abdominal organs, such as the appendix, cecum, ascending colon, and ovaries [4].
Preoperative diagnosis remains a quite challenging issue because affected patients usually present with nonspecific features of intestinal obstruction and most clinicians are not aware of the possibility of this disease. Therefore, more than half of cases are only diagnosed after laparotomy [2]. This report presents 2 cases of SEP with a recurrent small bowel obstruction that were treated successfully. In our cases, we doubted the preoperative diagnosis based on typical CT features, and confirmed the diagnosis by laparotomy.
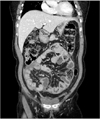
A 38-year-old man presented with a 24-hour history of lower abdominal pain. He had a history of recurrent attacks of abdominal pain once or twice a year, but they resolved spontaneously. The patient had no history of previous abdominal surgery or contributory conditions except psoriasis. The physical examination showed mild abdominal distension with periumbilical tenderness, but he was afebrile and hemodynamically stable. Contrast-enhanced CT showed a small bowel obstruction in the midabdomen with congregated loops to the center of the abdomen encased by a thickened peritoneum (Figs. 1, 2). Findings of laboratory studies were a white blood cell count of 12,900/mL (neutrophil, 78.7%) and a C-reactive protein of 5.5 mg/L. We first decided to manage the patient with conservative treatment, including nil per os, nasogastric tube decompression, and total parenteral nutrition. However, there was no improvement and we performed exploratory laparotomy.
At laparotomy, the entire small intestine was encapsulated by a thick membrane forming a cocoon-like lump. The fibrous membrane was excised with adhesiolysis and the small bowel loops were released. The histopathologic examination of the membranous tissue was consistent with peritoneal fibrosis. He had an uneventful postoperative period and was discharged on postoperative day seven. He took tamoxifen 20 mg once daily for one year. Now, he has no signs of recurrence until 28 months after surgery.
A 56-year-old woman was admitted to Daegu Catholic University Medical Center with a 2-month history of abdominal pain and constipation. She had diabetes, but no history of previous abdominal surgery or medications. The patient had been evaluated with esophagogastroduodenoscopy and colonoscopy 1 month ago, but the results were unremarkable. The physical examination showed mild abdominal distension with periumbilical tenderness. Laboratory studies showed a white blood cell count of 11,500/mL (neutrophil, 81.2%) and a C-reactive protein level of 32.2 mg/L. Contrast-enhanced CT showed small bowel loops that conglomerated at the midline and were encased by a dense peritoneal thickening.
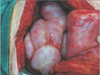
At laparotomy, the entire small intestine and colon were found encased in a cocoon-like fibrotic tissue (Figs. 3, 4). We performed adhesiolysis and the fibrotic membrane was freed from the bowels. Circulation of the bowel was intact. The appendix was found distended in the retrocecal area, and we performed an appendectomy. Histopathologic examination of the peritoneal biopsy revealed fibrosis with chronic inflammation suggestive of SEP. On postoperative day 8, the patient was discharged. Four months after surgery, the patient presented to the outpatient department with complaints of dyspnea on exertion. She was also experiencing a mild fever with productive cough since 1 week ago. On clinical examination, auscultation of the chest revealed poor air entry into the right lower hemi-thorax of the lung. Chest X-ray and CT scan of the chest showed right-sided pleural effusion. The pleural fluid was drained under ultrasound guidance and sent for analysis and culture. Biochemical evaluation of the pleural fluid revealed a pH of 7.6, glucose 245 mg/dL, protein 5.2 g/dL, lactate dehydrogenase 273 U/L, and adenosine deaminase (ADA) 51 U/L with lymphocytic predominance. PCR was negative for Mycobacterium tuberculosis. No growth was detected by the conventional aerobic culture method and the acid-fast bacillus was negative. Based on the clinical signs and symptoms and elevated ADA level, the patient was diagnosed with tubercular pleural effusion and given antituberculosis treatment. Her symptoms improved and subsequent visits were uneventful for 6 months until now.
SEP is a chronic inflammatory process in which the small intestines are encased by a dense fibrocollagenous membrane leading to acute or chronic intestinal obstruction [123]. This condition was described by Owtschinnikow in 1907, and was called peritonitis chronica fibrosa incapsulata [23]. SEP is classified as primary (idiopathic) or secondary, depending on the underlying cause. The primary or idiopathic form can be defined as SEP with no underlying cause, which was called "abdominal cocoon syndrome" historically [23].
Primary SEP classically presents in young girls living in tropical and subtropical regions, but recently cases in adult men in temperate zones have also been reported [12]. To explain the etiology, a number of hypotheses including retrograde menstruation with a superimposed viral infection and retrograde peritonitis and cell-mediated immunological tissue damage incited by gynecological infections have been proposed [123]. However, there seems to be little support for these theories because this disease has affected men, premenopausal women, and children. Some authors have argued that omental hypoplasia may be a probable etiology [5].
Secondary SEP is more common, and includes all causes that can generate chronic inflammation in the peritoneum. These include abdominal surgery, abdominal tuberculosis, peritoneal dialysis, ventriculoperitoneal or peritoneovenous shunts, liver transplantation, recurrent peritonitis, beta-blocker treatment (practolol or propranolol), intraperitoneal chemotherapy, intraperitoneal povidone-iodine use, liver cirrhosis, gastrointestinal malignancy, fibrogenic foreign material, systemic lupus erythematosus, and parasitic infection (sometimes leading to granulomatous peritonitis) [236]. Therefore, making a correct preoperative diagnosis in affected patients requires a comprehensive medical history. In the present report, the second case was finally demonstrated to have pulmonary tuberculosis four months after surgery. However, we could not demonstrate the suggestive findings of granulomatous peritonitis nor the existence of acid-fast bacilli in peritoneal biopsy.
Clinically, patients with SEP present with recurrent episodes of acute, subacute, or chronic small bowel obstruction, weight loss, malnutrition, nausea and anorexia. Sometimes there is also a palpable abdominal mass [4]. These clinical manifestations are nonspecific. Therefore, preoperative diagnosis of SEP is quite difficult, and many cases are only diagnosed after laparotomy. According to one extensive review of 149 cases, 43.6% of patients with SEP were diagnosed as abdominal cocoon syndrome preoperatively, while the majority of the remaining patients underwent operations for an initial diagnosis of intestinal obstruction and/or abdominal mass [2].
Contrast-enhanced CT is the most helpful radiologic modality for the preoperative diagnosis of SEP. The characteristic CT features are the appearance of small bowel segments that are conglomerated at the midline and encased by a dense capsule with a contrast-free periphery. Other findings include peritoneal thickening, loculated fluid collections, peritoneal calcification, marked enhancement of the peritoneum, tethering and thickening of the small bowel, and calcification over the liver capsule, spleen, posterior peritoneal wall, and bowel [7]. Furthermore, recent technological advances such as multiplanar reconstructed images provides more accurate tools for gauging the severity and other potential causes of intestinal obstruction, planning of surgical treatment, and avoiding unnecessary resection.
Abdominal cocoon is categorized into 3 types according to the extent of the encasing membrane that covers the intestine. Partial encapsulation of the intestine by a fibrocollagenous membrane is called type I abdominal cocoon syndrome. Complete coverage of the entire intestine by the membrane is called type II. Type III cocoon syndrome refers to encasement of the entire intestine, as well as other intra-abdominal organs, such as the appendix, cecum, ascending colon, and ovaries [4].
Histopathologic findings are not specific to SEP, but show fibrin deposition, fibroblast swelling (enlargement), capillary angiogenesis, mononuclear cell infiltration, and the presence of several immunohistochemical markers for peritoneal fibroblast activation and proliferation. These findings provide useful information when combined with surgical findings [8].
There is no evidence-based recommendation regarding the optimal treatment approach for patients with SEP. However, surgery remains the most effective management option for SEP, although there is controversy regarding surgical indications, optimal timing, approach methods, and procedures. The most suitable procedure that has been recommended is peritoneal decortication, which includes stripping the covering membrane off the intestinal surface and lysis of the dense adhesions between the intestinal loops until the loops lie free [9]. Every effort must be made to avoid iatrogenic bowel injury during this process. Resection is usually unnecessary, and when resection is performed without a solid indication, it may increase patient morbidity and mortality. Instilling an antiadhesive substance (e.g., sodium hyaluronate) between the intestinal loops before closing the abdomen may prevent the development of adhesive small bowel obstructions [4].
Patients with mild obstructive symptoms may be treated with medical therapy, including steroids, tamoxifen, and immunosuppressants [10]. Steroids are thought to inhibit collagen synthesis and maturation by suppressing the inflammatory process within the peritoneal membrane. They also completely eliminate the thickened membrane. Tamoxifen is a selective estrogen receptor modulator that inhibits fibroblastic production of transforming growth factor beta, a probiotic cytokine. This drug is therefore commonly used to treat certain fibrosclerotic disorders, such as retroperitoneal fibrosis and Riedel's thyroiditis [10]. However, there are limited data on the use of these medications in patients with primary SEP.
In conclusion, idiopathic SEP is a clinical entity of unknown cause that is characterized by encasement of the intestines by a fibrocollagenous cocoon-like membrane. Although recent advances in CT devices that allow for multiplanar imaging have enabled preoperative diagnosis of SEP, most cases are still incidentally diagnosed during laparotomy. Surgery remains the gold standard treatment for symptomatic idiopathic SEP. The most commonly used surgical method is membrane excision coupled with adhesiolysis. A better understanding of this condition and its radiological features should improve its management.
Figures and Tables
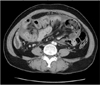
Fig. 1
The axial section shows marked dilatation and thickening of the small intestine, encased in a thick enhancing membrane. Fluid collection between the bowel loops is seen.
References
1. Foo KT, Ng KC, Rauff A, Foong WC, Sinniah R. Unusual small intestinal obstruction in adolescent girls: the abdominal cocoon. Br J Surg. 1978; 65:427–430.
2. Akbulut S. Accurate definition and management of idiopathic sclerosing encapsulating peritonitis. World J Gastroenterol. 2015; 21:675–687.
3. Tannoury JN, Abboud BN. Idiopathic sclerosing encapsulating peritonitis: abdominal cocoon. World J Gastroenterol. 2012; 18:1999–2004.
4. Wei B, Wei HB, Guo WP, Zheng ZH, Huang Y, Hu BG, et al. Diagnosis and treatment of abdominal cocoon: a report of 24 cases. Am J Surg. 2009; 198:348–353.
5. Xu P, Chen LH, Li YM. Idiopathic sclerosing encapsulating peritonitis (or abdominal cocoon): a report of 5 cases. World J Gastroenterol. 2007; 13:3649–3651.
6. Devay AO, Gomceli I, Korukluoglu B, Kusdemir A. An unusual and difficult diagnosis of intestinal obstruction: The abdominal cocoon. Case report and review of the literature. World J Emerg Surg. 2008; 3:36.
7. George C, Al-Zwae K, Nair S, Cast JE. Computed tomography appearances of sclerosing encapsulating peritonitis. Clin Radiol. 2007; 62:732–737.
8. Honda K, Oda H. Pathology of encapsulating peritoneal sclerosis. Perit Dial Int. 2005; 25:Suppl 4. S19–S29.
9. Singh B, Gupta S. Abdominal cocoon: a case series. Int J Surg. 2013; 11:325–328.
10. Habib SM, Betjes MG, Fieren MW, Boeschoten EW, Abrahams AC, Boer WH, et al. Management of encapsulating peritoneal sclerosis: a guideline on optimal and uniform treatment. Neth J Med. 2011; 69:500–507.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download