Abstract
Purpose
To investigate the feasibility and safety of solo surgery with single-port laparoscopic appendectomy, which is termed herein solo-SPLA (solo-single-port laparoscopic appendectomy).
Methods
This study prospectively collected and retrospectively analyzed data from patients who had undergone either non-solo-SPLA (n = 150) or solo-SPLA (n = 150). Several devices were utilized for complete, skin-to-skin solo-SPSA, including a Lone Star Retractor System and an adjustable mechanical camera holder.
Results
Operating times were not significantly different between solo- and non-solo-SPLA (45.0 ± 21.0 minutes vs. 46.7 ± 26.1 minutes, P = 0.646). Most postoperative variables were also comparable between groups, including the necessity for intravenous analgesics (0.7 ± 1.2 ampules [solo-SPLA] vs. 0.9 ± 1.5 ampules [non-solo-SPLA], P = 0.092), time interval to gas passing (1.3 ± 1.0 days vs. 1.4 ± 1.0 days, P = 0.182), and the incidence of postoperative complications (4.0% vs. 8.7%, P = 0.153). Moreover, solo-SPLA effectively lowered the operating cost by reducing surgical personnel expenses.
Solo surgery is a concept of an operation driven by a single surgeon without human assistant(s). The concept of solo surgery was first introduced [12] to overcome the difficulties arisen from the dissociation of operator's eye and hand in the laparoscopic surgery. In solo surgery, the self-controlled instrument holder replaces human assistant(s), enabling an operation without surgical personnel. The instrument holder makes it possible to guide the laparoscope according to the operator's own intention. Recent reports on solo surgery are rare, possibly due to (1) the complexity of installing and adjusting the devices and (2) ensuring adequate surgical personnel.
Along with advances in the instruments and techniques of the laparoscopic surgery, single-port laparoscopic surgery (SPLS) has been introduced. SPLS has also changed the numbers and the individual roles of participating surgical personnel [3]. For instance, SPLS usually requires two surgical members; the operating surgeon governs the entire operative process through bimanual manipulation, while an assistant guides the laparoscopic camera with minimal movement.
We believe that the recent surroundings driven by SPLS have made solo surgery more feasible and attractive. Furthermore, there are the issues of limited human resources in a number of countries (i.e., Korea, Taiwan, Canada) [456]. We herein performed single-port laparoscopic appendectomy (SPLA) by way of solo surgery, and termed it solo-SPLA. We herein intended to determine the safety and feasibility of solo-SPLA by comparing surgical outcomes with those of non-solo-SPLA series.
This study analyzed a prospectively collected database containing data from patients who underwent appendectomy due to appendicitis between April 2013 and February 2015 at the Department of Surgery, Daejeon St. Mary's Hospital, of Korea. The study was approved by the Ethics Committee at Daejeon St. Mary's Hospital (IRB number: DC14RISI0051).
In this study, non-solo-SPLA refers to SPLA performed in the presence of a human assistant, while solo-SPLA is defined as SPLA with only scrub nurse assistance. We first performed solo-SPLA in March 2013; since then, all the patients requiring appendectomy underwent solo-SPLA. Therefore, the inclusion criteria for non-solo-SPLA or solo-SPLA were identical and included all types of appendicitis regardless of its perforation. Exclusion criteria for either non-solo-SPLA or solo-SPLA included suspicious malignancy, American Society of Anesthesiologists physical status classification of IV or V, severe medical illness such as recent history of myocardial infarction, or refusal to participate in the study. All the series of operations (non solo- and solo-SPLAs) were performed by a single surgeon who had experienced more than 1,000 non-solo-SPLAs. The human assistants participating in non-solo-SPLA were mostly interns or physical assistant nurses.
Each patient was asked to self-assess their abdominal pain between postoperative day (POD) 0 (2 hours after the appendectomies), POD 1, and POD 2, using a visual analog scale (VAS), where a score of 0 indicated no pain and a score of 10 indicated the worst pain imaginable. We defined several terms for clarification. Uncomplicated appendicitis refers to the appendicitis prior perforation, and complicated appendicitis refers to the appendicitis which experienced its perforation, including perforated appendicitis and periappendiceal abscess. Regarding postoperative complications, urinary retention was defined as the need for prolonged catheterization (≥5 days) or reinsertion of a Foley catheter because of an inability to void. Intestinal obstruction was defined as the need for a nasogastric tube for 10 days or the need to reinsert a nasogastric tube after an oral diet was initiated [7].
Under general anesthesia, patients were placed in a supine position with the monitor on the right-hand side of the patient, opposite the surgeon (Fig. 1). After preparation, we made an approximately 1.0-cm-long vertical incision to the umbilicus and dissected to the peritoneum. At this time, we used a Lone Star Retractor System (3307G, Cooper Surgical, Trumbull, CT, USA) with 3-mm sharp disposable hooks (3311-8G, Cooper Surgical) to facilitate better visualization of the fascial and peritoneal layers of the umbilicus (Fig. 2). Under direct vision, the peritoneum was entered through the transverse fascial incision.
A single-port device was then introduced through the transumbilical incision. We initially used a homemade single port consisting of a wound retractor and a surgical glove [8]. More recently, we have used a commercial glove port (431AT-2W, Nelis, Bucheon, Korea) for convenience. After placement of the single port, the abdomen was insufflated with CO2 to a pressure of 12 mmHg. At this time, we installed the mechanical camera holder (Enodworld LAP53 Holding Systems, Karl Storz, Tuttlingen, Germany) by anchoring it to the operating table rail. A standard laparoscopic 5-mm camera (Full HD Laparoscope 5 mm, Aesculap, Tuttlingen, Germany) was then attached the camera holder and adjusted to provide the best surgical view (Fig. 3). The appendectomy procedure was performed with bimanual manipulation using a 5-mm grasper (Endo Grasp, Covidien, Mansfield, MA, USA) in the left hand and a 5-mm dissector (Endo Dissect, Covidien) in the right hand.
Most of the operative details of solo-SPLA were similar to those used during non-solo-SPLA. Briefly, after identifying the appendix, the mesoappendix was divided by either electrocauterization or clipping. The appendix was then ligated using an endoloop (Surgitie Ligating Loop, Covidien) and divided. The resected appendix was placed in a specimen retrieval bag (Lapbag, Sejong Medical Co., Paju, Korea) and extracted through the single port. If perforation with peritonitis or an abscess had occurred, extensive irrigation was performed. A Jackson-Pratt drain was placed via the umbilicus, if necessary. After abdominal deflation, intraumbilical fascial defects and transumbilical skin incisions were closed with interrupted sutures.
Perioperative management followed a standard protocol. All patients initially received instant intravenous hydration using crystalloid fluids and cefotetan (1.0 g/day to a maximum dose of 2.0 g/day). Since cefotetan has a 24-hour dosing schedule, it was administered intravenously before and after the operation for 2–3 days, and longer if needed. When perforation of the appendix had occurred, we coadministered intravenous metronidazole (50 mg/kg to a maximum dose of 2 g/day). Antibiotics were dose-adjusted or changed when there was no improvement in clinical parameters, including body temperature, leukocyte counts, or C-reactive protein levels. All patients were allowed a clear liquid diet upon their return to the ward, and the diet was advanced when it could be tolerated. Patients received intravenous ketorolac (0.1 mg/kg), as needed for pain, and were discharged when they tolerated a regular diet without evidence of complications. No oral antibiotics were administered at discharge, except for those who had experienced perforated appendicitis or periappendiceal abscess.
Numeric data are presented as means and standard deviations or as medians and ranges. Continuous variables were analyzed using the independent t-test, and categorical variables or proportions were compared using Pearson chisquare or Fisher exact tests, as appropriate. For variables with nonnormal distributions, Wilcoxon rank-sum tests were utilized to examine differences in the central tendencies. All P-values were two-tailed. Statistical analyses were performed using SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA). P-values < 0.05 were considered statistically significant.
Our study included a total of 300 patients with a median age of 37.0 years (range, 5–94 years) who underwent either non-solo-SPLA or solo-SPLA for appendicitis during the study period (Table 1). During the study period, no patients were excluded based on our exclusion criteria. The patients comprised 149 women (49.7%) and 151 men (50.3%). Of these, 232 and 68 patients (77.3% and 22.7%) exhibited uncomplicated and complicated appendicitis, respectively. In relation to admission routes, 269 patients (89.7%) were admitted via the Emergency Department and 31 (10.3%) were admitted via outpatient clinics. Preoperative variables, including age, sex, body mass index, comorbidities, and histories of prior laparotomies, were similar between both groups (Table 1).
We compared intraoperative and postoperative variables between the 2 groups (Table 2). The solo-SPLA group was similar to the non-solo-SPLA group in the proportions of uncomplicated to complicated appendicitis (P = 0.334) and histological grades (P = 0.073). Solo-SPLA did not prolong the operative time compared to non-solo-SPLA (45.0 ± 21.0 minutes vs. 46.7 ± 26.1 minutes, P = 0.646). To examine the solo-SPLA learning process, we investigated the change in operating times over time (initial 50, middle 50, and last 50 patients) (Fig. 4). The times steadily decreased, though without statistical significance (53.0 ± 19.9, 49.7 ± 21.1, and 45.9 ± 17.3 minutes, P = 0.264).
VAS scores for postoperative pain (Fig. 5) were also compared between groups. From the postoperative date to POD 2, there were no statistically significant differences in VAS scores. There was also no difference in the requirement for intravenous analgesics (0.7 ± 1.2 ampules [solo-SPLA] vs. 0.9 ± 1.5 ampules [non-solo-SPLA], P = 0.092). The solo-SPLA group showed similar postoperative variables to the non-solo-SPLA group, in terms of time to gas passing (1.3 ± 1.0 days vs. 1.4 ± 1.0 days, P = 0.182) and incidence of postoperative complications (4.0% vs. 8.7%, P = 0.153). Interestingly, the solo-SPLA group had shorter lengths of stay (LOS) than the non-solo-SPLA group (2.1 ± 1.8 days vs. 2.7 ± 2.1 days, P = 0.006).
In this study, we found that solo-SPLA did not prolong operating time compared to non-solo-SPLA. Solo-SPLA was also comparable to non-solo-SPLA in most postoperative variables, including postoperative VAS score, requirement for intravenous analgesics, time to gas passing, and incidence of postoperative complications. Moreover, solo-SPLA effectively lowered operating costs by reducing surgical personnel expenses. The reduced LOS in the solo-SPLA group is likely a reflection of recent emphasis on reducing LOS in our institution. Taken together, these results suggest that solo-SPLA is as safe and feasible as non-solo-SPLA, while reducing total operating costs.
Solo-SPLA is a hybrid operation that combines SPLA and solo surgery. This combination appears to compensate for the disadvantages of each to a large degree. The major drawback of solo surgery is discomfort due to the complexity of preparing more than two instrument holders. Solo-SPLA reduces this discomfort by requiring only one camera holder. Solo-SPLA also overcomes the limitations of SPLA by providing a wide space for an operating surgeon and a self-controlled operative vision.
Solo surgery offers cost savings. Generally, the laparoscopic approach requires greater costs related to longer procedure times and more expensive equipment [9]. Total operation costs are composed of material and personnel expenses. Solo-SPLA reduces operative expenses by limiting personnel expenses. Personnel expenses account for approximately 50% of the total cost [10]. Appendectomy usually requires 4–6 personnel, including members belonged to surgery, nursing, and anesthesiology department. Therefore, supposing that the contribution of the human assistant on the personnel expenses is 20%–30%, solo-SPLA will have the effect of lowering 10%–15% of total costs. Although this staff-saving effect does not directly lead to increased hospital revenue, solo-SPLA paves the way for more efficient utilization of human resources. Declining residency applications to surgical departments have resulted in manpower shortages in a number of hospitals in Korea, Taiwan, Canada, and other countries [456]. Under such shortages, solo-SPLS could make it possible to accommodate larger numbers of operations in regions with limited manpower.
Interestingly, solo-SPLS did not prolong operation time in this study. Solo-SPLA is naturally thought to increase operating time, primarily due to the use of limited manpower, and secondarily due to extra time consumed during the preparation and manipulation of the camera holder. Our result is in line with previous publications of reporting the operating time in solo surgery [11]. This observation was largely attributed to the stable visual field and reduced camera movements [11213]. In most uncomplicated appendicitis series, we fixed the camera after finding the appendix, and did not change the camera position until performing appendectomy. After appendectomy, we usually did 2 or 3 repositions for irrigation and exploration. Repositioning took less than three seconds each. Our mechanical holder required two hands to reposition.
One significant disadvantage of laparoscopic surgery is the dissociation between the operator's eye and hand; laparoscopic surgery provides an operating field that is controlled by a human assistant. This can lead to unsatisfactory interactions between operating and assistant surgeons, which may compromise the optimal visual field. Solo-SPLS removes this possibility by offering solo surgeon-driven camera adjustment. In a previous study, 9 surgeons completed a questionnaire after performing 1,033 traditional laparoscopic procedures using a joystick-guided camera holder (SoloAssist, Aktormed, Barbing, Germany) [14]. Eight of 9 surgeons preferred robotic to human assistance, mostly because of the steady image and camera control. Solo-SPLA provides this steady image and camera control using the lower-priced, but similarly effective mechanical camera holder.
In this study, the Lone Star Retractor System allowed a peritoneal opening without a human assistant. This system is a self-retaining retractor originally designed for improved visualization of anal procedures such as anorectal anastomoses [15]. To facilitate the view of fascial and peritoneal layers, we employed the retractor during the process of making a peritoneal opening. The Lone Star Retractor System thus enabled us to perform complete, skin-to-skin solo surgery.
Solo-SPLA has several disadvantages. The most significant disadvantage is the potential lag time between the appearance of an emergency and surgical management. It raises the importance of surgical systems which must be able to handle unexpected emergencies. For instance, operating room personnel could temporarily manage emergency situations before surgical personnel arrive. Next, solo-SPLA would restrict the opportunity for resident training, because it removes the necessity of a human assistant. However, there are still a variety of ways–such as actual performance of solo-SPLA under supervision of a practicing surgeon–in which residents could obtain surgical skills in the solo surgery environment. Finally, surgical decision-making in solo-SPLA could be inferior to that of non-solo-SPLA because solo-SPLA precludes discussion with an assistant about operative situations.
This study had several limitations. This retrospective review had a relatively small number of patients, and the findings should therefore be confirmed by prospective trials with larger patient populations. Selection bias cannot be completely avoided in all retrospective studies. We attempted to minimize this bias by assigning all non-solo-SPLA prior to assigning solo-SPLA. Accordingly, we could avoid selection bias occurred during the selection of solo-SPLA patients. The solo-SPLA series included earlier experiences on the learning curve. However, the surgical outcomes of the solo-SPLA group were comparable to the non-solo-SPLA group. Therefore, we believe that our results could be a manifestation of the operative feasibility of solo-SPLA.
In conclusion, despite the expected inconvenience arising from the operator's burden, solo-SPLA provided similar surgical outcomes as non-solo-SPLA. Specifically, solo-SPLA did not prolong operating time compared to non-solo-SPLA, and postoperative variables were comparable between procedures, including postoperative VAS scores, requirement for intravenous analgesics, time interval to gas passing, and incidence of postoperative complications. We believe that these results are likely due to the operative feasibility of solo-SPLA, which enables operator hand-to-eye coordination. Moreover, solo-SPLA could economize staff numbers, thereby helping to reduce health care costs. Thus, we believe solo-SPLA to be a reasonable alternative to non-solo-SPLA for those surgeons who can competently perform SPLA.
Figures and Tables
Fig. 1
Comparison of non-solo-SPLA and solo-SPLA operative settings for appendicitis. Operative setting for non-solo-SPLA (A) and operative illustration of solo-SPLA (B). SPLA, single-port laparoscopic appendectomy.

Fig. 2
Utilization of a Lone Star Retractor System (3307G, Cooper Surgical, Trumbull, CT, USA) for umbilical access for single-port insertion.
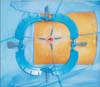
Fig. 3
Preparation and performance of solo-SPLA. (A) After single-port placement, a standard 5-mm laparoscopic camera was attached to the mechanical cameral holder. (B) The operation was performed by a single surgeon after a stable visual field had been acquired using the camera holder. SPLA, single-port laparoscopic appendectomy.
Fig. 4
Chronological sequence of operation times in patients who underwent solo-SPLA. Periods 1, 2, and 3 corresponds to the initial 50, middle 50, and the last 50 patients who underwent solo-SPLA. SPLA, single-port laparoscopic appendectomy.

Fig. 5
Comparison of non-solo-SPLA and solo-SPLA postoperative visual analog scale (VAS) scores. There were no significant differences in VAS scores between the two groups. SPLA, single-port laparoscopic appendectomy; POD, postoperative day.

References
1. Arezzo A, Ulmer F, Weiss O, Schurr MO, Hamad M, Buess GF. Experimental trial on solo surgery for minimally invasive therapy: comparison of different systems in a phantom model. Surg Endosc. 2000; 14:955–959.
2. Schurr MO, Buess GF. Systems technology in the operating theatre: a prerequisite for the use of advanced devices in surgery. Minim Invasive Ther Allied Technol. 2000; 9:179–184.
3. Kim SJ, Lee SC. Technical and instrumental prerequisites for single-port laparoscopic solo surgery: state of art. World J Gastroenterol. 2015; 21:4440–4446.
4. Chen YC, Shih CL, Wu CH, Chiu CH. Exploring factors that have caused a decrease in surgical manpower in Taiwan. Surg Innov. 2014; 21:520–527.
5. Deedar-Ali-Khawaja R, Khan SM. Trends of surgical career selection among medical students and graduates: a global perspective. J Surg Educ. 2010; 67:237–248.
6. Marschall JG, Karimuddin AA. Decline in popularity of general surgery as a career choice in North America: review of postgraduate residency training selection in Canada, 1996-2001. World J Surg. 2003; 27:249–252.
7. Kronberg U, Kiran RP, Soliman MS, Hammel JP, Galway U, Coffey JC, et al. A characterization of factors determining postoperative ileus after laparoscopic colectomy enables the generation of a novel predictive score. Ann Surg. 2011; 253:78–81.
8. Kim SJ, Ryu GO, Choi BJ, Kim JG, Lee KJ, Lee SC, et al. The short-term outcomes of conventional and single-port laparoscopic surgery for colorectal cancer. Ann Surg. 2011; 254:933–940.
9. Biondi A, Grosso G, Mistretta A, Marventano S, Toscano C, Drago F, et al. Laparoscopic vs. open approach for colorectal cancer: evolution over time of minimal invasive surgery. BMC Surg. 2013; 13:Suppl 2. S12.
10. Shoemaker P, Schuhmann TM. Trends in hospitals' use of contract labor. Healthc Financ Manage. 2007; 61:70–74.
11. Gillen S, Pletzer B, Heiligensetzer A, Wolf P, Kleeff J, Feussner H, et al. Solo-surgical laparoscopic cholecystectomy with a joystick-guided camera device: a case-control study. Surg Endosc. 2014; 28:164–170.
12. den Boer KT, Bruijn M, Jaspers JE, Stassen LP, Erp WF, Jansen A, et al. Time-action analysis of instrument positioners in laparoscopic cholecystectomy. Surg Endosc. 2002; 16:142–147.
13. Voorhorst FA, Meijer DW, Overbeeke CJ. Head-controlled laparoscopy: experiment, prototype, and preliminary results. J Laparoendosc Adv Surg Tech A. 1999; 9:379–388.
14. Hollander SW, Klingen HJ, Fritz M, Djalali P, Birk D. Robotic camera assistance and its benefit in 1033 traditional laparoscopic procedures: prospective clinical trial using a joystick-guided camera holder. Surg Technol Int. 2014; 25:19–23.
15. Gaujoux S, Bretagnol F, Au J, Ferron M, Panis Y. Single port access proctectomy with total mesorectal excision and intersphincteric resection with a primary transanal approach. Colorectal Dis. 2011; 13:e305–e307.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download