Abstract
Purpose
Intraductal papillary mucinous neoplasm (IPMN) has variable malignant potential ranging from premalignant intraductal lesions to malignant neoplasms with invasive carcinoma. To help physicians managing patients with IPMN, International consensus guidelines was made in 2006 and revised in 2012. This study was designed to evaluate the clinical usefulness of guidelines and to validate.
Methods
From October 1996 to December 2011, we retrospectively reviewed the data of 230 patients who underwent pancreatic resection for IPMN. Univariate and multivariable analyses were used to identify significant predictors of malignancy in IPMN.
Results
Of the 230 patients, 62 patients (27%) were diagnosed with invasive carcinoma. Jaundice (P < 0.001; 95% confidence interval [CI], 3.086–40.010) main pancreatic duct diameter equal to or greater than 10 mm (P < 0.001; 95% CI, 1.723–6.673) and also abdominal pain (P < 0.001; 95% CI, 4.363–22.600) show statistical significance in univariate and multivariate analysis. "High-risk stigmata" was statistical powerful predictors of malignancy than "worrisome features". International consensus guidelines 2012 had improvement on specificity but deterioration of sensitivity.
Noninflammatory cystic lesions of the pancreas are more common than previously recognized and being diagnosed with increasing frequency [1]. In autopsy study, small cystic lesions were found in nearly half of the 300 patients studied, the prevalence increasing with age [2]. It is therefore not surprising that with the increasing use of high-resolution abdominal imaging techniques, cystic neoplasms of the pancreas are being increasingly identified often as incidental findings [3].
Intraductal papillary mucinous neoplasm (IPMN) has variable malignant potential ranging from premalignant intraductal lesions to malignant neoplasms with invasive carcinoma. Compared to noninvasive IPMN, invasive cancers confer a distinct worse prognosis, with a 5-year overall survival (OS) of 36%–70% [45]. Clinically, IPMN is classified into three types according to the involvements of pancreatic ducts: main duct (MD) IPMN, branch duct (BD) IPMN, and mixed type IPMN. The malignancy risk of BD-IPMN, MD-IPMN and mixed type IPMN is 24.4%, 62.2%, and 57.6%, respectively [6].
To help physicians managing patients with cystic neoplasms of the pancreas, international consensus guidelines for IPMN and MCN of pancreas was made in 2006 [7]. Subsequent studies have been performed to identify factors predicting malignancy and indications for surgical resection of IPMN, especially the BD type because it is most common variant of this disease, has the lowest risk of malignancy, and is most often diagnosed incidentally [8]. And a large number of series validating the safety of this approach have been published [9101112131415161718] although some have reported risk of cancer in small BD-IPMN of up to 20% [192021]. It resulted in a second set of international consensus guidelines, published in 2012 [6]. After new guidelines were published, many studies have been done to prove how effective the guidelines are. But yet, it is still debatable [22232425]. This study was designed to evaluate the clinical usefulness and to validate international consensus guidelines 2012 (ICG2012).
This study included consecutive 230 patients who underwent surgery for IPMN at Samsung Medical Center from October 1996 to December 2011. For the purpose of this study, this database was retrospectively analyzed and supplemented with a review of electronic medical records.
IPMN was diagnosed by contrast-enhanced CT, MRI/magnetic resonance cholangiopancreatography. Lesions were classified into 3 types: MD, BD, and mixed type based on international consensus guidelines 2006 (ICG2006). They were reclassified into 3 groups: "high risk stigmata", "worrisome features", "no criteria" based on ICG2012. Then compare the results according to each guidelines. The number of high-risk stigmata was expressed as "HRS score". Patients with any of the high risk stigmata were classified into the "high risk stigmata" group. Patients with HRS score of 0 were assessed for worrisome features, with the number of worrisome features expressed as "WF score". Patients with WF scores of 1 or more were classified into the "worrisome features" group and those with WF scores of 0 were classified into "no criteria" group.
"High-risk stigmata" include obstructive jaundice (serum total bilirubin > 1.5 mg/dL and lesion in pancreas head), enhanced solid component, and dilation of the main pancreatic duct (MPD) to a diameter greater than 10 mm with surgical resection strongly recommended for patients with any of these stigmata. In contrast, worrisome features include the 6 factors: history of pancreatitis, maximal cyst diameter greater than 30 mm, thickened and enhanced cyst walls, MPD diameter 5 to 9 mm, nonenhanced mural nodules, abrupt change in the caliber of the MPD with distal pancreatic atrophy. We also include abdominal pain, CEA level, CA 19-9 level, age, sex then analyzed each clinical variables to figure out what could predict malignancy.
IPMN was diagnosed according to the 2010 World Health Organization criteria. It is categorized as low, intermediate or high grade; other lesions were described as IPMN with associated invasive carcinoma. In this study, low, intermediate grade dysplasias were classified as benign and high grade dysplasia and IPMN with associated invasive carcinoma as malignancy.
All statistical analyses were performed using IBM SPSS ver. 18.0 (IBM Co., Armonk, NY, USA). Results were expressed as mean ± standard error and median with range. Comparisons between 2 groups were assessed using the chi-square test, Fisher exact probability test, or the Mann-Whitney U-test, as appropriate. Risk factors were validated by logistic regression test. disease-free survival (DFS) and OS were assessed using Kaplan-Meier method. Differences were considered significant when P < 0.05.
The demographic and clinical characteristics of the 230 identified patients are shown in Table 1. Median patient age was 63.0 years (range, 32–85 years) and male to female ratio was 1.98:1. Median CEA level was 2.8 ng/mL (range, 0.16–119.50 ng/mL) and median CA 19-9 level was 209.1 U/mL (range, 0.1–43,983.9 U/mL). Of 230 tumors, 130 (56.5%) were located in head of pancreas. Mean cyst size was 3.6 cm and mean MPD size was 0.58 cm. BD type IPMNs were 114 (49.6%) and Pylorus-preserving pancreatoduodenectomy was the most performed procedure (86 patients, 37.4%), followed by distal pancreatectomy (72 patients, 31.3%) and Whipple operation (33 patients, 14.3%). Most common pathology type was low or moderate grade dysplasia (153 patients, 66.5%), followed by invasive carcinoma (62 patients, 27%) and high grade dysplasia and noninvasive carcinoma (15 patients, 6.5%) (Table 1).
Table 2 shows the diagnostic significance of clinical value of "worrisome features" and "high-risk stigmata" for predicting malignancy. None of clinical value of "worrisome features' shows statistical significance for predicting malignancy. Otherwise, two of three clinical values of "high-risk stigmata" shows statistical significance for predicting malignancy, jaundice (P < 0.001; 95% CI, 3.086–40.010) and main pancreatic duct diameter greater than 10 mm (P < 0.001; 95% CI, 1.723–6.673). And also abdominal pain (P < 0.001; 95% CI, 4.363–22.600) shows statistical significance. With multivariable analysis, those 3 factors also show statistical significance (jaundice: P < 0.001; 95% CI, 4.403–70.982; MPD > 10 mm: P = 0.005; 95% CI, 1.428–7.365; abdominal pain: P < 0.001; 95% CI, 5.266–32.427) (Table 3).
Table 4 shows influence of HRS scores and WF scores on the prediction of malignancy. Patients with any of clinical value of "worrisome features" don't have statistical significance. Only "WF scores = 2" group shows statistical significance (P = 0.022; 95% CI, 1.166–7.046). Otherwise patients with "high-risk stigmata" shows statistical significance for predicting malignancy (P < 0.001; 95% CI, 2.138–6.895) and cumulative in risk prediction as there is stepwise increase.
Presence of mural nodule did not have statistically significance for predicting malignancy in our study. But many studies showed mural nodule could be one of powerful malignancy predicting factor [1617]. For further evaluation, we made 3 subgroups of patients with mural nodule depends on size (Table 5). Nevertheless, none of subgroups showed statistically significance for predicting malignancy.
Figs. 1 and 2 show sensitivity and specificity of ICG2006 and ICG2012. When we used ICG2006, sensitivity was 90%, specificity was 34%. Positive predictive value (PPV) and negative predictive value (NPV) was 41% and 87%. Otherwise with ICG2012, sensitivity was 55%, specificity was 78%. PPV and NPV was 55% and 78%.
Figs. 3 and 4 show DFS and OS between "with guidelines" group and "without guidelines" group for each guidelines. "With guidelines" group is in accord with guideline and "without guidelines" group is not in accord with guidelines. Median follow-up period was 39.4 months. Even though the only statistically powerful result was comparison DFS between "with ICG2012" and "without ICG2012", all DFS and OS were lower in "with guidelines" group. It could infer patients who were treated by guidelines had more severe disease progression indirectly.
The main purpose of this study was to figure out how effective and useful revised new guidelines, published 2012, are. Furthermore we tried to figure out which clinical variable could predict malignancy. Our results confirm that updated guidelines seemed to bring improvement of weak side of ICG2006. However updated guidelines need more supplementation.
A natural history study estimated the 5-year actuarial riskfor BD-IPMN progressing to high grade dysplasia to be 15%, which was significantly lower than that observed for MD-IPMN . Other study shows that those who managed with observation based on ICG2012, over median follow-up for 5 years, 21% required surgical resection [26]. Considering other results of studies, many of patients with IPMN can be managed with observation, so it is required to make precise diagnosis of malignancy to avoid unnecessary surgeries. In this study, statistical significant clinical variables predicting malignancy were abdominal pain (P < 0.001), jaundice (P < 0.001), MPD diameter greater than 10 mm (P < 0.001). Other studies said that cyst size greater than 3 cm, mural nodules, CA 19-9 greater than 37 U/mL, age, sex showed statistical significance [2426], but our study showed there were no statistical significance.
After ICG2006 was published, many studies had been performed and found several characteristics. One of the characteristics of ICG2006 was high sensitivity and relative low specificity [111215]. Our study had a sensitivity and specificity of 90%, 34% respectively. However, application of ICG2012, we had a sensitivity and specificity of 55%, 78%. New guidelines had improvement on specificity but not on sensitivity. In general, ICG2012 was less stringent in recommending resection for IPMN compared with ICG2006 and proposed surveillance for a greater proportion of IPMN. Criteria such as the presence of symptoms and pancreatic juice cytology which have been shown to be associated with malignancy [27], have been removed from the updated guidelines, allowing many patients with symptoms and elevation of CEA in pancreas juice without "worrisome" or "high risk stigmata" features to be observed [28]. More studies are needed to improve both sensitivity and specificity.
There are some limitations to this study. First, many of patients did not get endoscopic ultrasonography - fine needle aspiration (EUS-FNA) so that it is limited to evaluate surgical indications of ICG2012. Pancreatic juice cytology potentially has important roles to determine the adequate treatment choice in patients with IPMN with "worrisome features", and to detect significant lesions that could not be detected by other imaging modalities [27]. CEA concentrations greater than 30 ng/mL in pancreatic juice were found to differentiate between nonmalignant and malignant IPMN with sensitivity, specificity, and accuracy of 94%, 85%, and 90%, respectively [29]. Carcinoembryonic antigen concentrations greater than 200 ng/mL in cyst fluid collected by EUS-FNA had sensitivity, specificity, PPV, and NPV for the diagnosis of malignant IPMN of 90%, 71%, 47%, and 96% [30]. The addition of EUS-FNA to abdominal imaging such as CT and MRI significantly increase overall accuracy for diagnosis of neoplastic pancreatic cysts. From now we need to perform EUS to diagnosis and treat IPMN for reducing unnecessary surgery. Second, this study was designed as retrospective evaluation of data, analysis of only patients who underwent surgery. Comparing with those who managed with observation, we may find better outcomes.
In conclusion, revised guidelines seemed to bring about an improvement of weak side of Sendai criteria. Bur it still needs supplementation to improve accuracy for predicting malignancy of IPMN. In our study, abdominal pain, jaundice, main pancreas duct greater than 10 mm can be clinical variables to predict malignancy.
Figures and Tables
Fig. 1
Classification of IPMN patients based on International Consensus Guideline 2006. IPMN, intraductal papillary mucinous neoplasm. Sensitivigy, 90%; Specificity, 34%; positive predictive value, 41%; negative predictive value, 87%.
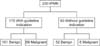
Fig. 2
Classification of IPMN patients based on International Consensus Guideline 2012. IPMN, intraductal papillary mucinous neoplasm. Sensitivigy, 55%; Specificity, 78%; positive predictive value, 55%; negative predictive value, 78%.
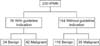
Fig. 3
(A) Disease-free survival between "With ICG2006" group and "Without ICG2006" group. (B) Disease-free survival between "With ICG2012" group and "Without ICG2012" group. ICG, international consensus guideline.

Fig. 4
(A) Overall survival between "With ICG2006" group and "Without ICG2006" group. (B) Overall survival between "With ICG2012" group and "Without ICG2012" group guideline 2012. ICG, international consensus guideline.

References
1. Fernandez-del Castillo C, Adsay NV. Intraductal papillary mucinous neoplasms of the pancreas. Gastroenterology. 2010; 139:708–713. 713.e1–713.e2.
2. Kimura W, Nagai H, Kuroda A, Muto T, Esaki Y. Analysis of small cystic lesions of the pancreas. Int J Pancreatol. 1995; 18:197–206.
3. Fernandez-del Castillo C, Targarona J, Thayer SP, Rattner DW, Brugge WR, Warshaw AL. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003; 138:427–423.
4. Grutzmann R, Post S, Saeger HD, Niedergethmann M. Intraductal papillary mucinous neoplasia (IPMN) of the pancreas: its diagnosis, treatment, and prognosis. Dtsch Arztebl Int. 2011; 108:788–794.
5. Swartz MJ, Hsu CC, Pawlik TM, Winter J, Hruban RH, Guler M, et al. Adjuvant chemoradiotherapy after pancreatic resection for invasive carcinoma associated with intraductal papillary mucinous neoplasm of the pancreas. Int J Radiat Oncol Biol Phys. 2010; 76:839–844.
6. Tanaka M, Fernandez-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012; 12:183–197.
7. Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006; 6:17–32.
8. Brugge WR, Lauwers GY, Sahani D, Fernandez-del Castillo C, Warshaw AL. Cystic neoplasms of the pancreas. N Engl J Med. 2004; 351:1218–1226.
9. Akita H, Takeda Y, Hoshino H, Wada H, Kobayashi S, Marubashi S, et al. Mural nodule in branch duct-type intraductal papillary mucinous neoplasms of the pancreas is a marker of malignant transformation and indication for surgery. Am J Surg. 2011; 202:214–219.
10. Maguchi H, Tanno S, Mizuno N, Hanada K, Kobayashi G, Hatori T, et al. Natural history of branch duct intraductal papillary mucinous neoplasms of the pancreas: a multicenter study in Japan. Pancreas. 2011; 40:364–370.
11. Nagai K, Doi R, Ito T, Kida A, Koizumi M, Masui T, et al. Single-institution validation of the international consensus guidelines for treatment of branch duct intraductal papillary mucinous neoplasms of the pancreas. J Hepatobiliary Pancreat Surg. 2009; 16:353–358.
12. Pelaez-Luna M, Chari ST, Smyrk TC, Takahashi N, Clain JE, Levy MJ, et al. Do consensus indications for resection in branch duct intraductal papillary mucinous neoplasm predict malignancy? A study of 147 patients. Am J Gastroenterol. 2007; 102:1759–1764.
13. Rodriguez JR, Salvia R, Crippa S, Warshaw AL, Bassi C, Falconi M, et al. Branchduct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resection. Gastroenterology. 2007; 133:72–79.
14. Salvia R, Crippa S, Falconi M, Bassi C, Guarise A, Scarpa A, et al. Branch-duct intraductal papillary mucinous neoplasms of the pancreas: to operate or not to operate? Gut. 2007; 56:1086–1090.
15. Tang RS, Weinberg B, Dawson DW, Reber H, Hines OJ, Tomlinson JS, et al. Evaluation of the guidelines for management of pancreatic branch-duct intraductal papillary mucinous neoplasm. Clin Gastroenterol Hepatol. 2008; 6:815–819.
16. Tanno S, Nakano Y, Nishikawa T, Nakamura K, Sasajima J, Minoguchi M, et al. Natural history of branch duct intraductal papillary-mucinous neoplasms of the pancreas without mural nodules: long-term follow-up results. Gut. 2008; 57:339–343.
17. Uehara H, Ishikawa O, Katayama K, Kawada N, Ikezawa K, Fukutake N, et al. Size of mural nodule as an indicator of surgery for branch duct intraductal papillary mucinous neoplasm of the pancreas during follow-up. J Gastroenterol. 2011; 46:657–663.
18. Woo SM, Ryu JK, Lee SH, Yoon WJ, Kim YT, Yoon YB. Branch duct intraductal papillary mucinous neoplasms in a retrospective series of 190 patients. Br J Surg. 2009; 96:405–411.
19. Jang JY, Kim SW, Lee SE, Yang SH, Lee KU, Lee YJ, et al. Treatment guidelines for branch duct type intraductal papillary mucinous neoplasms of the pancreas: when can we operate or observe? Ann Surg Oncol. 2008; 15:199–205.
20. Sawhney MS, Al-Bashir S, Cury MS, Brown A, Chuttani R, Pleskow DK, et al. International consensus guidelines for surgical resection of mucinous neoplasms cannot be applied to all cystic lesions of the pancreas. Clin Gastroenterol Hepatol. 2009; 7:1373–1376.
21. Walsh RM, Vogt DP, Henderson JM, Hirose K, Mason T, Bencsath K, et al. Management of suspected pancreatic cystic neoplasms based on cyst size. Surgery. 2008; 144:677–684.
22. Aso T, Ohtsuka T, Matsunaga T, Kimura H, Watanabe Y, Tamura K, et al. "High-risk stigmata" of the 2012 international consensus guidelines correlate with the malignant grade of branch duct intraductal papillary mucinous neoplasms of the pancreas. Pancreas. 2014; 43:1239–1243.
23. Goh BK, Thng CH, Tan DM, Low AS, Wong JS, Cheow PC, et al. Evaluation of the Sendai and 2012 International Consensus Guidelines based on cross-sectional imaging findings performed for the initial triage of mucinous cystic lesions of the pancreas: a single institution experience with 114 surgically treated patients. Am J Surg. 2014; 208:202–209.
24. Jang JY, Park T, Lee S, Kang MJ, Lee SY, Lee KB, et al. Validation of international consensus guidelines for the resection of branch duct-type intraductal papillary mucinous neoplasms. Br J Surg. 2014; 101:686–692.
25. Roch AM, Ceppa EP, DeWitt JM, Al-Haddad MA, House MG, Nakeeb A, et al. International Consensus Guidelines parameters for the prediction of malignancy in intraductal papillary mucinous neoplasm are not properly weighted and are not cumulative. HPB (Oxford). 2014; 16:929–935.
26. Sahora K, Mino-Kenudson M, Brugge W, Thayer SP, Ferrone CR, Sahani D, et al. Branch duct intraductal papillary mucinous neoplasms: does cyst size change the tip of the scale? A critical analysis of the revised international consensus guidelines in a large single-institutional series. Ann Surg. 2013; 258:466–475.
27. Ohtsuka T, Matsunaga T, Kimura H, Watanabe Y, Tamura K, Ideno N, et al. Role of pancreatic juice cytology in the preoperative management of intraductal papillary mucinous neoplasm of the pancreas in the era of international consensus guidelines 2012. World J Surg. 2014; 38:2994–3001.
28. Goh BK, Lin Z, Tan DM, Thng CH, Khor CJ, Lim TK, et al. Evaluation of the Fukuoka Consensus Guidelines for intraductal papillary mucinous neoplasms of the pancreas: Results from a systematic review of 1,382 surgically resected patients. Surgery. 2015; 158:1192–1202.
29. Hirono S, Tani M, Kawai M, Okada K, Miyazawa M, Shimizu A, et al. The carcinoembryonic antigen level in pancreatic juice and mural nodule size are predictors of malignancy for branch duct type intraductal papillary mucinous neoplasms of the pancreas. Ann Surg. 2012; 255:517–522.
30. Maire F, Voitot H, Aubert A, Palazzo L, O'Toole D, Couvelard A, et al. Intraductal papillary mucinous neoplasms of the pancreas: performance of pancreatic fluid analysis for positive diagnosis and the prediction of malignancy. Am J Gastroenterol. 2008; 103:2871–2877.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download