INTRODUCTION
Laparoendoscopic single-site donor nephrectomy
Patient and methods
Results
SURGICAL TECHNIQUE
A lateral white line of Toldt was dissected, and descending colon was mobilized medially.
The gonadal vein was dissected from the level of the iliac vessels up to the lower pole of the kidney, ligated, and divided near the renal hilum.
During posterior dissection of the renal vein, the lumbar vein was dissected and divided.
The adrenal vein was dissected and divided at the upper border of the left renal vein.
After circumferential dissection of the renal vein, dissection was continued medially to the origin of the abdominal aorta.
The renal artery was identified and dissected circumferentially.
The ureter was dissected downward to beneath the level of the iliac vessels.
The entire kidney was freely mobilized by dissecting the lateral and posterior aspects.
A gynecologist performed a posterior colpotomy via intraabdominal approach.
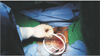
After introducing a medium-sized wound retractor via a vaginal incision, a large wound retractor was inserted through the medium one and overlapped (Fig. 1).
The surgical glove was fixed to the outer ring of the large wound retractor to maintain pneumoperitoneum.
After making a small incision in the fingertip portion of the glove, a 15-mm trocar was inserted.
The distal side of the ureter was ligated using a 5-mm Hemolock (Hemolock Ligating Clips, Teleflex, Morrisville, NC, USA). Then, the ureter was resected with an endoshear, and urine outflow was checked.
A Lap-bag (Sejong Medical Co., Paju, Korea) was introduced transvaginally, and the kidney including the ureter was placed in the Lap-bag.
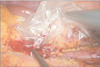
The renal artery was ligated close to the aorta with a Multifire Endo-TA stapler (Auto Suture 30 mm–2.5 mm; Covidien, Mansfield, MA, USA) and cut distally with an endoshear.
Similarly, the renal vein was also ligated and divided as close to the inferior vena cava as possible with the Multifire Endo-TA stapler (Auto Suture 30–2.5 mm, Covidien) and endoshear (Fig. 2).
The Lap-bag orifice was closed, and the kidney was delivered transvaginally.
The assistant removed the kidney from the bag, avoiding contamination from the external side of the bag.
The colpotomy was repaired transvaginally with interrupted full-thickness reabsorbable sutures and checked at the intraperitoneal site with the laparoscope.
Intra-abdominal conditions including hemostasis were checked laparoscopically, and the descending colon was reperitonealized with a V-loc wound closure device (Covidien).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download