Abstract
Major peripheral arterial graft infection is a potentially devastating complication of vascular surgery, associated with significant mortality and high amputation rates. Autologous saphenous veins are considered optimal arterial conduits for lower extremity revascularization in infected fields, but they are often unavailable or unsuitable in these patients. This study describes two patients with major peripheral graft infection, but without available autologous veins, who underwent graft excision and cryopreserved cadaveric arterial allograft reconstruction. Although long-term graft durability is unclear because of gradual deterioration and degeneration, these findings suggest that cadaveric allografts may be good options for patients with major peripheral graft infection.
Major peripheral graft infection in patients with severe underlying peripheral arterial occlusive disease is associated with high operative mortality, recurrent infection, and amputation rates, especially when autologous conduits are unavailable or unsuitable [1,2,3]. Prosthetic vascular grafts are not the most suitable for vascular reconstructions, because of their higher susceptibility to infection. Although cryopreserved cadaveric arterial allografts are regarded as a feasible alternative, long-term graft durability is unclear because of gradual graft deterioration and degeneration [3].
This report represents the clinical outcomes of two patients with major peripheral arterial graft infection who underwent cryopreserved cadaveric arterial allograft reconstruction.
A 49-year-old, chronic alcoholic male with a previous medical history of end-stage renal disease due to lupus nephritis (World Health Organization class IV) and who had been receiving hemodialysis for the past 3.5 years through a left forearm arteriovenous fistula, as well as having dilated cardiomyopathy and atrial fibrillation, was admitted to Asan Medical Center because of massive painful swelling of the right thigh and high fever. Eight days prior to admission, the patient had complained of a sudden onset of painful, spontaneous bulging in the medial aspect of his right thigh. Contrast-enhanced dynamic CT scan at another facility showed rupture of the superficial femoral artery (SFA) with a large hematoma in the proximal medial thigh. He underwent an emergency SFA interposition bypass with the ipsilateral great saphenous vein at the outside facility. Five days later, however, massive bleeding recurred due to rupture of the anastomosis, and he underwent a second SFA interposition bypass with the contralateral great saphenous vein. Three days after the second operation, the patient was transferred to our hospital because of continued bleeding at the operated site and a high fever.
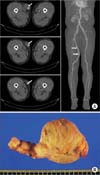
Initial laboratory tests showed elevated serum white blood cell count (30,700/mm3) and C-reactive protein (26.70 mg/dL) and myoglobin (1,401 ng/mL) levels, and decreased hemoglobin level (6.2 g/dL). A contrast-enhanced CT scan showed rupture of the right SFA with active extravasation and diffuse calcification of the lower extremity arteries (Fig. 1A). An emergency operation was performed. Intraoperative examination revealed skin and muscle necrosis, intense perivascular inflammation, and destruction of the interposed vein wall, suggestive of gross perivascular tissue infection (Fig. 1B). Since the patient lacked an available or suitable autologous vascular conduit, a cryopreserved cadaveric iliac artery was used for SFA interposition bypass after complete debridement of the infected tissue, including the SFA itself and the interposed vein graft (Fig. 1C). Excisional biopsy revealed acute necrotizing inflammation with dystrophic calcification and thrombus in the SFA and acute necrotizing inflammation with numerous bacterial colonies, especially methicillin-resistant Staphylococcus aureus (MRSA) and Acinetobacter baumannii, in the vein graft. Cultures of blood samples drawn at admission were negative for bacteria. After continued antimicrobial therapy and debridement, an anterolateral thigh free flap was performed on postoperative day 14 (Fig. 1D). The patient was discharged on postoperative day 48 without any complications.
During follow-up, the interposition bypass graft remained patent, and there was no evidence of recurrent arterial allograft infection. However, the patient died suddenly of cardiac arrest due to dilated cardiomyopathy 3 years after the operation.
A 55-year-old male with a previous medical history of chronic massive deep vein thrombosis involving the infrarenal inferior vena cava due to hypercoagulability and treatment with oral anticoagulants for 12.5 years was admitted to our hospital because of a 1-month history of resting pain in the right leg. One month prior to admission, the patient was diagnosed at another institution with acute occlusion of the right above-knee popliteal artery, but thrombectomy at this facility was unsuccessful.
Since this patient did not have an available or suitable autologous vascular conduit, he underwent right femoropopliteal arterial bypass with a prosthetic graft (GORE-TEX, W. L. Gore & Associates Inc., Newark, DE, USA). Although no immediate postoperative complications were observed, recurrent graft infections developed 1 and 4 months after surgery, and he presented with high fever (38.9℃). Blood and wound cultures were positive for MRSA. After continued antimicrobial therapy, the infected tissue, including the segment of the prosthetic graft, was completely debrided, and cryopreserved cadaveric iliac arterial allograft reconstruction was performed. Because antibiotic treatment had resulted in elevated serum creatinine concentrations, postoperative imaging was performed using Duplex ultrasonography, which showed patent flow in the femoro-popliteal arterial bypass graft. The patient was discharged without any complications.
During follow-up, the patient did well with a patent bypass graft and no evidence of recurrent arterial allograft infection. However, a CT scan performed 4 years after surgery showed huge aneurysmal changes of the cadaveric allograft, suggestive of its deterioration and degeneration (Fig. 2A). Since there was no evidence of recurrent arterial allograft infection, a new prosthetic graft (GORE-TEX) was implanted, and the cadaveric arterial allograft was resected (Fig. 2B). Histologic evaluation of the resected allograft revealed that the arterial wall was replaced by fibrosis especially in the muscular layer and mixed diffuse inflammatory cell infiltration was noted. He was discharged without any complications. To date, there have been no further signs of graft infection.
Major peripheral arterial graft infection is a rare but severe and potentially life-threatening complication of vascular surgery, associated with significant mortality and high amputation rates [1,2,3]. The basic goals of treatment of these patients are the eradication of infection and the maintenance of adequate perfusion for limb salvage. However, the optimal management of major peripheral graft infection is still unclear. Autologous saphenous veins are considered the optimal arterial conduits for lower extremity revascularization in infected fields, but they are often unavailable or unsuitable in these patients. Prosthetic vascular grafts, although recently developed, are not the most suitable material for vascular reconstruction because of their higher susceptibility to infection. Although cryopreserved cadaveric arterial allografts may be a feasible alternative for treating this vascular complication, long-term graft durability is unclear because of gradual deterioration and degeneration [3].
Arterial allografts were the first widely used vessel grafts in the 1950s. However, they are no longer used clinically because of their high incidence of complications and low patency rates [4,5]. Arterial allografts are antigenic and elicit immune rejection, resulting in gradual deterioration and degeneration of the grafts [6]. Although experimental data in animals suggest that a low-maintenance dose of cyclosporine provides effective immunosuppression, thus preventing aneurysmal changes in the arterial allograft, cyclosporine can also have serious adverse effects in elderly, critically ill patients [7]. Cryopreservation is generally regarded as useful for the storage of vascular tissue, but the optimal cryopreservation methods have not been determined. Several studies have shown that advanced methods of cryopreservation maintain the function of an arterial allograft close to that of a normal artery, with other studies reporting improved clinical outcomes with arterial allografts preserved using advanced methods in patients with major peripheral arterial graft infection [8,9]. Thus, cryopreserved arterial allografts have been considered useful vascular conduits in patients contraindicated for use of a prosthetic graft, including patients with active infection or contamination of the surgical site and lacking available vein material [10].
The traditional treatment for major peripheral arterial graft infection consists of total graft excision with revascularization. However, the operative management of this potentially life-threatening vascular complication presents technical challenges to the vascular surgeon, especially in patients without available or suitable autologous vascular conduits. This study described the clinical outcomes in two patients with major peripheral arterial graft infection who underwent cryopreserved cadaveric arterial allograft reconstruction. One patient presented with a patent graft during the 3-year follow-up period, whereas the other required implantation of a new prosthetic graft after 4 years due to aneurysmal changes in the cadaveric allograft. These findings suggest that cadaveric allografts may be a good option for the treatment of patients with major peripheral graft infection. Although long-term graft durability is unclear, reconstruction with a cryopreserved arterial allograft should be regarded as a safe, if temporary, method of eradicating infection, permitting subsequent reconstruction with prosthetic material when necessary.
Figures and Tables
 | Fig. 1Findings in case 1. (A) Preoperative contrast-enhanced CT scan, showing rupture of the right superficial femoral artery (white arrow) with active extravasation. (B) Intraoperative findings included skin and muscle necrosis and intense perivascular inflammation with destruction of the interposed vein wall (white arrows). (C) After complete debridement of the infected tissue, a cryopreserved cadaveric iliac artery (white arrows) was used for superficial femoral artery interposition bypass. (D) On postoperative day 14, an anterolateral thigh free flap was performed and a follow-up photograph was taken before discharge. |
References
1. McCready RA, Bryant MA, Divelbiss J, Wack MF, Mattison HR. Case study: chronic femoropopliteal prosthetic graft infection with exposed graft. Vasc Endovascular Surg. 2009; 43:291–294.
2. Reilly LM, Stoney RJ, Goldstone J, Ehrenfeld WK. Improved management of aortic graft infection: the influence of operation sequence and staging. J Vasc Surg. 1987; 5:421–431.
3. Castier Y, Paraskevas N, Maury JM, Karsenti A, Cerceau O, Legendre AF, et al. Cryopreserved arterial allograft reconstruction for infected peripheral bypass. Ann Vasc Surg. 2010; 24:994–999.
4. Szilagyi DE, Mcdonald RT, Smith RF, Whitcomb JG. Biologic fate of human arterial homografts. AMA Arch Surg. 1957; 75:506–527.
5. Meade JW, Linton RR, Darling RC, Menendez CV. Arterial homografts: a long-term clinical follow-up. Arch Surg. 1966; 93:392–399.
6. Axthelm SC, Porter JM, Strickland S, Baur GM. Antigenicity of venous allografts. Ann Surg. 1979; 189:290–293.
7. Schmitz-Rixen T, Megerman J, Colvin RB, Williams AM, Abbott WM. Immunosuppressive treatment of aortic allografts. J Vasc Surg. 1988; 7:82–92.
8. Bia D, Pessana F, Armentano R, Perez H, Graf S, Zocalo Y, et al. Cryopreservation procedure does not modify human carotid homografts mechanical properties: an isobaric and dynamic analysis. Cell Tissue Bank. 2006; 7:183–194.
9. Chiesa R, Astore D, Piccolo G, Melissano G, Jannello A, Frigerio D, et al. Fresh and cryopreserved arterial homografts in the treatment of prosthetic graft infections: experience of the Italian Collaborative Vascular Homograft Group. Ann Vasc Surg. 1998; 12:457–462.
10. Kieffer E, Gomes D, Chiche L, Fleron MH, Koskas F, Bahnini A. Allograft replacement for infrarenal aortic graft infection: early and late results in 179 patients. J Vasc Surg. 2004; 39:1009–1017.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download