Abstract
Transumbilical single-port laparoscopic donor nephrectomy (SPLDN) is a novel, rapidly evolving, minimally invasive treatment modality for kidney transplantation. This method causes minimal parietal injury, has cosmetic advantages, and allows rapid recovery because of low postoperative pain and short hospital stay. Like other abdominal surgeries, when conducted by experienced laparoscopic surgeons, it can meet the same graft requirements as conventional laparoscopic surgery. Here, we report the first two cases of transumbilical SPLDN at Daejeon St. Mary's Hospital, The Catholic University of Korea. We used the umbilicus as a common path for laparoscopic procedures and as a route for specimen retrieval. The operating times were 230 and 265 minutes in cases 1 and 2, respectively. No intra- or postoperative complications were noted. In case 1, the wound length was 4 cm and duration of hospitalization was 2 days. In case 2, the wound length was only 2.5 cm, and the duration of hospitalization was only 1 day.
Conventional laparoscopic surgery, which was introduced in 1995, has resulted in substantial changes in surgical practice, and is now the primary choice for donor nephrectomy [1]. However, despite the generally excellent results achieved with conventional laparoscopic surgery, such as a short hospital stay, decreased use of narcotics, and low rates of wound complications, the need to minimize the extent of access for better cosmetic results and less pain remains [2]. Donor nephrectomy differs from other surgeries in that donors do not benefit from the operation. Therefore, considerable research efforts are being directed toward the development of natural orifice transluminal endoscopic surgery (NOTES) and single-port laparoscopic surgery (SPLS) to overcome these issues related to conventional laparoscopic surgery [3]. The feasibility of NOTES for donor nephrectomy has been demonstrated, but some problems that hamper its widespread clinical application persist. In contrast, transumbilical SPLS is at an early stage of development as a minimally invasive surgical method [4]. The umbilicus is located at the center of the abdomen, where the abdominal wall is the thinnest and no specific vessels or nerves are present. Through this inborn ready-made wound, most intraperitoneal organs and corners can be accessed easily. In addition, the surgical scar can easily be concealed postoperatively.
Unlike regular surgery, which focuses only on the patient, transplantation surgery is characterized by the emotional burden it places on the donor as well as the recipient, and the results of transplantation surgery must be successful for both parties. In cases of transplantation surgery, recipients usually feel apologetic toward donors. If the donor scar is bigger than assumed, recipients feel guiltier. Furthermore, donors also feel burdened or encumbered by large scars. Thus, the graft can greatly influence donors in Single-port laparoscopic donor nephrectomy (SPLDN), as it can make them less fearful of kidney donation [5].
At our institute, SPLS was first performed at the end of 2008, and since early 2009, it has been actively performed by a laparoscopic surgeon (S.C.L.) who is experienced in most fields of surgery. Thus far, this surgeon has conducted SPLS in over 1,800 cases for various diseases and conditions. Transumbilical pure SPLDN was performed in December 2013 at Daejeon St. Mary's Hospital, The Catholic University of Korea, as the first case of SPLDN in Korea.
For transumbilical pure SPLDN, we use the umbilicus as a common path for laparoscopic procedures and specimen retrieval, whereby possible peritoneal injury can be avoided, along with potential complications related to trocar insertion and the incision required for graft retrieval.
Here, we present the first two cases we experienced of this novel transumbilical SPLDN with graft retrieval.
The operation was performed using conventional straight and rigid laparoscopic instruments and a new device, namely, the Multi Endo-TA stapler (Covidien, Mansfield, MA, USA), which enabled optimal vascular control. We used the Endo-TA stapler for ligation of the renal artery and vein, and divided the vessels using lap-scissors without sealing the graft-side vessels. There are two advantages to this technique. One is that the graft size can be small, since some blood leaks out of the transected vessels. The other is that because the graft-side vessels are not stapled, 2-3 mm of the renal artery and vein of the graft are preserved. Furthermore, since the umbilicus has a reverse triangular shape and is anatomically invaginated, if the umbilical fold is stretched, the length of the vertical umbilicus increases to almost twice the length in the nonstretched position. Thus, we can make a 1.5-2.0 cm longer umbilical incision that still appears small.
The patient under general anesthesia was placed in the right semilateral position by inserting and fixing a cushion at the left lower bedside and tilting the bed to the left. The patient was thus in the supine position when the operation was initiated. This was changed to the right-side decubitus position, that is, the supine position with the right arm adducted, when the port was installed and the bed was tilted to the right in order to obtain a surgical view of the intra-abdominal cavity. A 3.0- to 3.5-cm vertical umbilical incision was made and the patient's position was changed to the head-down position with the right side tilted down, and a commercial single port (OCTO port, Dalim, Seoul, Korea) was introduced into the peritoneum. The peritoneum was inspected with a 10-mm (30 degree) rigid laparoscope after carbon dioxide gas insufflation. A small piece of gauze with a radio-opaque marker was inserted into the abdominal cavity to serve as a protective barrier from thermal injury, a dam to prevent soiling from outside the operative field, and a brace or retractor to allow for better operative vision; It is particularly useful for atraumatic manipulation of the kidney. The other general procedures and sequences were the same as those used in conventional laparoscopic donor nephrectomy, as briefly described below (Fig. 1).
1. A lateral white line dissection is continued following the Toldt fusion fascia to the colon proper while verifying locations of the left ureter, gonadal vessels, and left kidney.
2. The gonadal vein is dissected and ligated at the level between the lower border of the left renal vein and the lower pole of the kidney in the area of the hylum.
3. The adrenal vein is dissected and ligated at the upper border of the left renal vein, and a dissection is made up to the plane between the spleen and the upper pole of the kidney.
4. A dissection is made medially to confluence of the renal vein to the inferior vena cava.
5. The posterior aspect of the renal vein is dissected, and the dissection is continued medially to the origin of the abdominal aorta while checking the accessory branches.
6. The ureter is dissected downward to beneath the level of the pelvic inlet almost to the pelvic-side wall.
7. The entire kidney is freely mobilized by dissecting the lateral and posterior aspects.
8. The distal side of the ureter is ligated using a 5-mm Hemolock (Hemolock Ligating Clips, Teleflex, Morrisville, NC, USA) only on the donor side. Then, it is resected with a shear and urine outflow is checked.
9. A Lap-bag (Sejong Medical Co., Paju, Korea) is inserted, and the kidney body including the ureter but not the renal vessels is placed in the Lap-bag. The vessel stalks on the hylum are left free around the orifice of the Lap-bag.
10. The renal artery is ligated as close to the aorta as possible by using the Multi Endo-TA stapler (Auto Suture 30-2.5 mm; Covidien) and cut distally with a shear.
11. Similarly, the renal vein is also ligated and divided as close to the inferior vena cava as possible with the Multi Endo-TA stapler and shear.
12. The orifice of the Lap-bag is closed transumbilically and the kidney is removed.
13. The kidney is placed in a prepared iced basin, and the back table procedure is initiated.
14. To continue the SPLS, intra-abdominal conditions including hemostasis are checked and the descending colon is replaced in its initial location.
15. The umbilical incision is closed and dressed.
The donor was a 30-year-old woman (body mass index [BMI], 29.26 kg/m2; height, 153 cm; weight, 68.5 kg). She had no history of previous surgery or underlying disease. The graft obtained was a left kidney graft measuring 11.0 × 6.0 × 3.0 cm (165 g). The length of the renal artery and vein was not measured, but no specific difficulties related to vessel length arose. The warm ischemic time was 3 minutes 8 seconds, and the total ischemic time was 48 minutes. The operating time was 230 minutes, and no intra- or postoperative complications were noted. The patient was discharged on postoperative day 2. The length of the umbilicus wound when she visited the outpatient clinic was 4 cm.
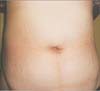
The donor was a 42-year-old woman (BMI, 27.72 kg/m2; height, 163.3 cm; weight, 73.3 kg). She had no history of previous surgery or underlying disease. A left kidney graft measuring 11.0 × 8.0 × 4.5 cm (198 g) was obtained. The length of the renal artery was 2.5 cm, and that of the renal vein was 4.4 cm. The warm ischemic time was 1 minute 22 seconds, and the total ischemic time was 47 minutes. The operating time was 265 minutes, and no intra- or postoperative complications were noted. The patient was discharged on postoperative day 1. The length of the umbilicus wound measured when she visited the outpatient clinic was 2.0 cm, and the wound was not easily identifiable (Fig. 2).
Demographics and operative/postoperative data of patients were shown in Table 1 and Table 2, respectively. Although the surgery was performed using the same method in both cases, the wound size and postoperative peritoneal restoration differed. In both cases, the vessel length was sufficient for vascular anastomoses and successful kidney transplantation using standard techniques. In addition, the recipients in both cases were discharged in good condition on posttransplant days 14 and 12, and they had good allograft function.
Laparoscopic nephrectomy is a useful alternative to open surgery. Its efficacy and safety have been demonstrated based on short-term outcomes in several studies, including multicenter studies [6,7]. Thus, laparoscopic surgery is being increasingly applied in donor nephrectomy. Under standard conditions, conventional laparoscopic surgery requires four ports and an additional lower abdominal incision for graft extraction. The increase in the number of incisions increases the likelihood of complications related to abdominal wall injuries, which may cause temporary pain, muscle spasms, bleeding, cutaneous nerve injury, etc. Wound infection and incisional hernias are also potential problems [8]. The comparative benefits of SPLDN are a better cosmetic outcome, less pain, and fewer incisions. Moreover, transumbilical SPLDN may allow common laparoscopic procedures to be performed entirely through the umbilicus and permit graft retrieval.
The kidney is a spongy organ in which absorbed blood is normally congested. If this blood is removed, the size and weight of the organ would reduce considerably and its elasticity would change. Furthermore, the kidney is generally an elongated structure, and umbilical incisions in our technique are performed in the longitudinal direction. If the umbilicus is viewed three-dimensionally from the lateral side, it appears like a triangle with sides of 1.5-2 cm. From the front, the incision length appears to be 1.5 cm while it is actually about 3 cm. Thus, in our novel technique, drainage of blood from the kidney reduces the graft size, because of which it can be removed through just a 4- to 5-cm incision. Furthermore, 1.5-2 cm of the umbilical incision are hidden.
Once vascular skeletonization of the kidney and optimal dissection are accomplished, a Lap-bag (Endo-catch bag) is placed in the abdomen, and the kidney, excluding the renal vessels, is placed in the Lap-bag; the renal vessels are finally cut. If the Lap-bag is removed as soon as the renal artery is cut, the warm ischemic time can be reduced. As mentioned, we used Endo-TA for handling the renal vessels; we placed double-line stapling on the non-graft-side renal vessels, but we freely cut the graft-side vessels, without using staples, endoclips, or Hemolock. This allowed blood to drain from the graft, causing graft size and volume to be considerably reduced (Fig. 3). Another advantage of this procedure is that an additional 3- to 5-mm margin of the renal vein became available. Overall, graft size was reduced and the kidney could easily be removed with this technique.
Despite these benefits, there remain some doubts regarding the application of SPLDN for graft harvesting. Many surgeons are concerned about the difficulty of graft retrieval and graft quality. Transumbilical graft retrieval is not difficult, because when the OCTO port is removed, only a 2-cm umbilical incision is needed; the rectus fasciotomy is then extended beneath the skin cranially and caudally, and the umbilical skin incision is extended to approximately 4 cm (Fig. 4). The pre-bagged specimen can then be gently extracted because of the hidden available 2 cm [9].
In the cases described in the present report, the Multi Endo-TA stapler was used for ligation of the renal artery and vein. With single side-double row stapling, this stapler does not leave staples on the graft side, and it yields an additional length of approximately 5 mm of the graft vessel compared to the Endo-GIA stapler [6].
Our surgical method yielded better results than those conducted at several other centers. For example, other SPLDN patients had an average transumbilical fascial incision length of 7.0 cm after renal extraction and an average apparent incision length of 3.8 cm after closure [10]. However, in our cases, the incision length and apparent length were only 4 and 2 cm, respectively. Furthermore, although the reported length of hospital stay is similar, the warm ischemic times differ considerably. While the warm ischemic time was 3 minutes 8 seconds and 1 minute 22 seconds in the present cases, other centers have reported warm ischemic times exceeding 5 minutes [10].
In conclusion, in our experience, SPLDN was safe and feasible. In addition to the cosmetic advantages, the principles of donor nephrectomy, including adequate warm ischemic time and sufficient vessel and ureter length, could be fulfilled entirely using SPLS. However, the technique warrants further experience and prospective randomized studies to ensure graft safety.
Figures and Tables
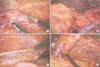
Fig. 1
Laparoscopic view. (A) Dissection and ligation of gonadal vein. (B) Dissection and ligation of adrenal vein. (C) Dissection and ligation of ureter. (D) Kidney mobilization.
References
1. Ramasamy R, Afaneh C, Katz M, Chen X, Aull MJ, Leeser DB, et al. Comparison of complications of laparoscopic versus laparoendoscopic single site donor nephrectomy using the modified Clavien grading system. J Urol. 2011; 186:1386–1390.
2. Afaneh C, Aull MJ, Gimenez E, Wang G, Charlton M, Leeser DB, et al. Comparison of laparoendoscopic single-site donor nephrectomy and conventional laparoscopic donor nephrectomy: donor and recipient outcomes. Urology. 2011; 78:1332–1337.
3. Kaouk JH, Khalifeh A, Laydner H, Autorino R, Hillyer SP, Panumatrassamee K, et al. Transvaginal hybrid natural orifice transluminal surgery robotic donor nephrectomy: first clinical application. Urology. 2012; 80:1171–1175.
4. Kaouk JH, Autorino R. Laparoendoscopic single-site surgery (LESS) and nephrectomy: current evidence and future perspectives. Eur Urol. 2012; 62:613–615.
5. Lunsford KE, Harris MT, Nicoll KN, Collins BH, Sudan DL, Kuo PC, et al. Single-site laparoscopic living donor nephrectomy offers comparable perioperative outcomes to conventional laparoscopic living donor nephrectomy at a higher cost. Transplantation. 2011; 91:e16–e17.
6. Kok NF, Lind MY, Hansson BM, Pilzecker D, Mertens zur Borg IR, Knipscheer BC, et al. Comparison of laparoscopic and mini incision open donor nephrectomy: single blind, randomised controlled clinical trial. BMJ. 2006; 333:221.
7. Simforoosh N, Basiri A, Tabibi A, Shakhssalim N, Hosseini Moghaddam SM. Comparison of laparoscopic and open donor nephrectomy: a randomized controlled trial. BJU Int. 2005; 95:851–855.
8. Meng MV, Freise CE, Kang SM, Duh QY, Stoller ML. Techniques to optimize vascular control during laparoscopic donor nephrectomy. Urology. 2003; 61:93–97.
9. Canes D, Berger A, Aron M, Brandina R, Goldfarb DA, Shoskes D, et al. Laparoendoscopic single site (LESS) versus standard laparoscopic left donor nephrectomy: matched-pair comparison. Eur Urol. 2010; 57:95–101.
10. Barth RN, Phelan MW, Goldschen L, Munivenkatappa RB, Jacobs SC, Bartlett ST, et al. Single-port donor nephrectomy provides improved patient satisfaction and equivalent outcomes. Ann Surg. 2013; 257:527–533.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download