Abstract
Purpose
Choledochoduodenal fistula (CDF) is an extremely rare condition even in the most populous nations. However, diagnostic tools are inadequate for the young surgeon to be made aware of such a rare condition before surgery. Hence, basic understanding of the epidemiology, etiology, and management for this unusual but discoverable condition are necessary and essential.
Methods
The exclusive case reports of CDF, which were published from 1983 to 2014 concerning mainland Chinese people, were performed to review the epidemiology, etiology, and management.
Results
A total of 728 cases were incorporated into this review among 48 papers. More than half of the CDF cases were female (416) with an average age of 57.3 years. CDF was usually caused by cholelithiasis (573 of 728). Epigastric pain (589 of 728) and cholangitis (395 of 728) were the most common symptoms of CDF. CDF was usually detected and confirmed by endoscopic retrograde cholangiopancreatography (ERCP) (475 of 728) in Mainland China. The fistulas larger than 1 cm (82 of 654) were recommended for surgical biliary reconstruction. Fistulas between 0.5 cm and 1.0 cm (467 of 654) which were followed frequently by cholangitis attacks also required surgery; the rest were recommended to have stone removal and/or the application of an effective biliary drainage. Fistulas less than 0.5 cm (105 of 654) were usually received conservative therapy.
Conclusion
CDF should be considered in differential diagnosis of recurrent epigastric pain and cholangitis. A possible ERCP should be arranged to investigate carefully. Depending on the size of fistula and clinical presentation, different programs for CDF are indicated, ranging from drug therapy to choledochojejunostomy.
Biliary-enteric fistula was first described by Bartholin in 1654, but so far biliary-enteric fistulas are still rarely reported, which are thought to be one or multiple pathological perforations between biliary tree and gastrointestinal tract [12]. Choledochoduodenal fistula (CDF), the special type of biliaryenteric fistulas, is nearly 90% caused by cholecystolithiasis [3]. Increasing cases have been reported in the last 30 years since the progress in hepatobiliary techniques, such as endoscopic retrograde cholangiography (ERCP), magnetic resonance cholangiopancreatography (MRCP), which have been applied to extensively reevaluate hepatobiliary diseases in the clinic, especially in Mainland China. However, the preoperative diagnosis of CDF is still difficult because of the nonspecific and/or minimal clinical symptoms [4]. Hence, CDFs are resulting in tough challenges for surgeons, especially for young surgeons. In this review, we have mentioned exclusive cases reports of CDF in Chinese populations concerning epidemiology, etiology, diagnosis and management.
The MeSH terms biliary fistula and intestinal fistula with the subheadings of China were searched in the English databases such as PubMed, Embase, ISI Web of Science and the Chinese databases such as SinoMed, CQVIP, CNKI, WANFANG data. A total of 1,379 papers have been researched. Cholecystoduodenal fistula was not encountered in discussions. Data on the papers were extracted from the full text. Papers were reviewed elaborately, except for those without specific reference or already reported. A total of 48 case reports from 1983 to 2014 concerning 728 cases of CDF were finally accorded with the inclusion criteria [5678910111213141516171819202122232425262728293031323334353637383940414243444546474849505152]. Statistical calculations have not been built as they are lacking in the heterogeneity of data.
Large-scale studies of ERCP have reported that the incidence of CDF ranges from 2.53% to 5.3% [2223242745]. More than half of the cases are found in females (57.14%). The ratio of male/female is 0.75. The mean age of the 728 patients is 48.9 ranging from 18 to 82. The case reports of CDF, which have accurate age data, totals 24 papers concerning 33 cases. In these papers, the mean age of male (n = 13) and female (n = 20) are respectively 48 and 57.3. The distribution of CDF in different districts of Mainland China is shown in Fig. 1.
CDF is a complication of cholelithiasis, including gallstone, choledocholithiasis, hepatolith, and biliary stenosisin, which account for 73.76% of CDF in all cases. The remainder of cases is rarely caused by iatrogenic injury (9.20%), spontaneity (8.93%), penetrating peptic ulcer (3.16%), adjacent organs tumors (3.58%), and abdominal tuberculosis (0.27%) and so on (Table 1).
Generally, no typical symptoms of CDF are presented. The most common presenting symptoms of nonobstructing biliary-enteric fistulas are epigastric pain (80.91%) and cholangitis such as jaundice (54.26%) and fever (50.69%), nausea and/or vomiting (10.30%), abdominal distension (0.39%), asymptomatic (0.11%), and diarrhea (0.11%). A case of melena and a case of kaolin stools have been respectively reported by Cao et al. [5] and Shen and Zhao [18]. Cachexia such as anorexia, ascites and weight loss commonly present in the advanced tumor patient [5192227304152] (Table 1).
ERCP is the most common and effective method for the diagnosis of CDF. In the 728 cases of CDF, 475 cases were confirmed by ERCP (65.25%), the rest were detected by operation (23.21%), X-barium meal examination (4.40%), T-tube cholangiography (3.43%), MRCP (1.79%), gastroscope/duodenoscope (1.37%), percutaneous cholangiography (0.41%), autopsy (0.14%) and so on. Under the detection of ERCP, surgery, gastroscope/duodenoscope, and the fistula size are classified as less than 0.5-cm group, more than 1.0-cm group and 0.5- to 1.0-cm group, respectively. The number of above groups is respectively 105 cases, 82 cases, and 467 cases. Ultrasound and CT have rarely been useful in CDF diagnosis (Table 1).
At present, treatments of CDF are controversial. In the 728 cases of CDF, patients treated by biliary reconstruction including resection of hepatic bile duct stricture or stricturoplasty side-to-side choledochojejunostomy, intrahepatic cholangiojejunostomy and transection of common bile duct (CBD) account for 46.84%. The rest were nonoperative treatment (14.01%), endoscopic sphincterotomy (EST) (13.74%), fistula repair surgery (11.95%), drainage of CBD (9.89%), subtotal gastrectomy (2.34%). Four cases were under vater papilla reconstruction [34]. Two cases suffered from fistulation [4647]. One case was implanted with a self-expandable metal stent in the CBD to heal the fistula [52] (Table 1).
In the West, the most common communication was cholecystoduodenal, but in Asia, choledochoduodenal was the most common type [53]. This may be related to physical endurance. Asians are prone to endure pain when they suffer from cholangitis or CBD stone, so CDF is more prevalent in Asian patients [54]. Especially, Southwest China's population more easily suffers from gallstone, choledocholithiasis, hepatolith, and biliary stenosisin than other regions of Mainland China [232427]. We also know from these reports above that women are prone to suffer from CDF, which may be due to a higher prevalence of cholelithiasis in women. In contrast, CDF historically has been a complication of duodenal ulcer disease and has been seen more often in men.
CDF is a well-known but relatively rare complication of duodenal ulcer in recent years, because the development of antacid drugs helps us control the disease more easily than before [55]. The major cause of duodeno-biliary fistula is inflammation of the bile duct due to gallstones, and minor causes include duodenal ulcer, pancreatic neoplasm, and inflammation of neighboring organs [56]. As we know, there are two parts of narrow in CBD, one part is of the duodenal papilla, and the other is the site of the bile duct in entering the duodenum. When stones impact on either of the two narrow parts and press against the wall of CBD, inflammation would occur repeatedly and necrosis will form eventually [57].
Perampullary adenocarcinoma or pancreatic carcinoma could lead to CDF when a tumor presses against the CBD directly, making it obstructive [58]. Then CBD enlarges as the pressure in bile duct increases, causing CBD to crack and finally a fistula formation [59]. The carcinoma could penetrate the local tissues and cause necrosis and fistula formation as well [60]. Duodenal ulcer could penetrate the intestinal and biliary walls into CBD and ultimately cause a fistula, which would usually occur in the site of the duodenal bulb. The final result is the iatrogenic factor, which can not be neglected. For example, when one patient undergoes exploration of CBD, ducts around the periampullary region could be injured during passage of a rigid choledochal bougie in the CBD. Furthermore, iatrogenic CDF could occur while performing sphincteroplasty or EST carelessly [6162]. In addition, previous studies also reported CDF formation in a patient with duodenal tuberculosis, liver transplantation and metallic biliary stent placement.
Moreover, perapapillary choledochoduodenal fistula (PCDF) is mainly caused by a spontaneous migration of a CBD stone into the duodenum. Karincaoglu et al. [63] reviewed 841 patients who underwent ERCP between 1993 and 2002 for evaluation of PCDF. They found that 16 had a PCDF at the papilla Vater and none of these 16 patients had a history of pancreatitis, duodenal ulcer, nor had they undergone biliary surgery or ERCP previously. This study indicated that PCDF was more frequently associated with CBD stones than with biliary surgery and bougienage.
CDF has been categorized by Ikeda and Okada [64] and Gong et al. [2324] separately. According to the location of the fistula, Ikeda divided CDFs into two types. Type I is located on the longitudinal fold of the papilla, while type II is on the posterior wall of the duodenal bulb (Fig. 2A). However, Gong et al [2324] have divided them into three types, the first type is type A, which is an orifice of CDF located more than 2 cm away from the papilla. The second is type B, characterized by an orifice of CDF located less than 2 cm away from the papilla. Lastly, type C, also called PCDF, is an orifice of CDF located on the papilla fold (Fig. 2B). The classification of CDF is important for the diagnosis and treatment in the clinic, and the position of fistula can suggest what the possible cause of CDF is.
The clinical manifestations of CDF are various and nonspecific. To determine the typical presentations of CDF, more specific clinical datum are needed. What is more, assistant examinations are necessary in the diagnosis. There are some signs related to these diseases. The presence of an internal biliary fistula was suggested in some cases by the findings of pneumobilia, atrophic gallbladder, and biliary stones [65]. Furthermore, the bile sample collected from the CBD showed high levels of pancreatic enzymes, including amylase and phospholipase-A2 [66]. High levels of these enzymes may indicate an abnormal connection between the duodenum and the CBD. Despite the increased rate of CDF diagnosis, treatment and management strategies for CDF have not been established yet, and more investigation is needed in this area.
Some studies suggest that patients with no symptoms of illness probably should not receive treatment, especially surgical. Treatment can sometimes be accomplished through nonsurgical (endoscopic) or percutaneous interventions. Even in patients with symptoms conservative treatment of H2 antagonist or proton pump inhibitors and drugs for the eradication of Helicobacter pylori infection (when abundant), which often happens, is advised. This therapy can also lead to less CDF [67686970]. Surgery is recommended when there are surgical complications, duodenal stenosis, severe symptoms of peptic ulcer disease in those who have not responded to conservative treatment, especially bleeding and recurrent cholangitis.
On the other hand, Choi et al. [71] have regarded CDF as a biliary abnormality that was prone to ascending biliary infection. To avoid recurrence of bile tract infection and the risk of biliary stricture existing after healing of the fistula, aggressive therapy to correct CDF was mandatory. In addition, recurrent gallstone ileus caused by CDF was considered as a definitive indication for surgical managements. What is more, CDF presented a high risk for biliary tract carcinoma, and surgical management to be necessary for CDF, even if the patient had no significant clinical symptoms [7273].
Agarwal et al. [74] have reported that most of their patients were operated on with biliary enteric anastomosis, and the remaining patients underwent other therapeutic procedures, such as sphincterotomy, biliary stenting, and nasobiliary drainage. According to the sizes of fistula, Li et al. [35] proposed the following strategies for fistula treatments: for fistula orifices larger than 1 cm, CBDs larger than 2 cm and with complications in the biliary tree, after removing the stones, Roux-en-Y choledochojejunostomy or Roux-en-Y intrahepatic cholangiojejunostomy to build an effective drainage, a transection of the CBD was applied to prevent the reflux of duodenal juice; for fistula orifices between 0.5 and 1.0 cm, CBDs greater than 2 cm without any biliary complications and reflux of duodenal juice, a side-to-side choledochojejunostomy without transecting the duct and an effective biliary drainage was applied; for fistula orifices larger than 0.5 cm, CBDs larger than 1.2 cm and CDF caused by secondary stones from the gallbladder, the surgery included stone removal, cholecystectomy and CBD examination followed by drainage; for fistula orifices less than 0.5 cm, without complications in the bile duct tree non-surgical treatments were applied. This study suggested that larger fistula increased the frequency of cholangitis episodes and needed surgical treatment for fistula itself. In many patients, when there was stenosis of the duodenum, it was recommended gastric resection.
Biliary-enteric fistula including CDF is one of the reasons for converting from laparoscopic cholecystectomy (LC) to open surgery. Thus, surgery is preferred over laparoscopic operation for treating CDF. However, as the skill of LC improves, LC would expand its indication [56]. Periselneris and Bong [75] have reported that no patient suffered from death or intraoperative complications when the laparoscopic approach to the CDF was performed. And Lee et al. [76] also reported three cases of biliary-enteric fistula including CDF treated successfully by laparoscopic surgery without any postoperative complication, eventually. We can know that laparoscopic surgery is an advanced technique and the treatment of choice for CDF irrespective of the preoperative diagnosis from these studies.
In a word, there are several treatment options for CDF. Management of CDF depends on their type and etiological severity of the disease and the general condition of each patient. In fistulas with complicating duodenal ulcers, medical management or surgery can be used. Surgery must be reserved for patients with poorly controlled or recurrent ulcer symptoms, major ulcer complications, such as perforation, hemorrhage, or obstruction, or exceptional cases with cholangitis or biliary obstruction. Endoscopic management and the extraction of a bile duct stone (if present) may be needed. There is a justified trend towards conservative management of iatrogenic bile duct perforations. The current case was also managed conservatively, by reducing the pressure gradient with T-tube drainage and antibiotics.
In conclusion, patients with CDF may have nonspecific symptoms, which make the diagnosis difficult. To assist in diagnosing CDF, imaging procedures are needed. And ERCP can increase the rate of CDF diagnosis significantly. Although the rate of diagnosis has increased, treatment and management plans for CDF have not been established. Managements of CDF depend on the types, etiology, severity of the diseases, and the general condition of each patient. Despite open surgery being preferred over laparoscopic surgery for treating CDF, laparoscopic surgery was reported effective for cases with CDF irrespective of the preoperative diagnosis. And to prove the effectiveness of laparoscopic surgery, more data are needed to be collected.
Figures and Tables
Fig. 1
The distribution of choledochoduodenal fistula (CDF) in different districts of Mainland Chinese. Distribution in 48 articles regarding 728 cases of CDF fistula published from 1983 to 2014.
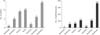
Fig. 2
The classification of choledochoduodenal fistula (CDF). (A) The Ikeda's classification. Type I was located on the longitudinal fold of the papilla, while type II was on the posterior wall of the duodenal bulb. (B) The Gong's classification. Type A is an orifice of CDF located more than 2 cm away from the papilla. Type B is an orifice of CDF located less than 2 cm away from papilla. Type C, or perapapillary CDF, is an orifice of CDF located on the papilla fold. 1, duodenum; 2, CBD; 3, pancreatic duct; 4, major duodenal papilla; 5, CDF.
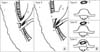
Table 1
The general conditions of cases
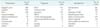
ERCP, endoscopic retrograde cholangiopancreatography; EST, endoscopic sphincterotomy; CBD, common bile duct; MRCP, magnetic resonance cholangiopancreatography; PTC, percutaneous transhepatic cholangiography.
a)A case of melena and a case of kaolin stools. b)Ultrasound and CT mainly indicated that air is present within the biliary system. c)One case is implanted a selfexpandable metal stent in the CBD to heal the fistula.
ACKNOWLEDGEMENTS
This study was supported by National Natural Science Foundation of China (No. 81071339).
References
1. Marshall T, Kamalvand K, Cairns SR. Endoscopic treatment of biliary enteric fistula. BMJ. 1990; 300:1176.
2. Liu TM, Chiu HH. Images in clinical medicine. Gallstone ileus. N Engl J Med. 2010; 362:345.
3. Lin CT, Hsu KF, Yu JC, Chu HC, Hsieh CB, Fu CY, et al. Choledochoduodenal fistula caused by cholangiocarcinoma of the distal common bile duct. Endoscopy. 2009; 41:Suppl 2. E319–E320.
4. Luu MB, Deziel DJ. Unusual complications of gallstones. Surg Clin North Am. 2014; 94:377–394.
5. Cao JY, Fang Z, Guo WT, Dai LH. A case report of choledochoduodenal fistula. Tianjin Yi Yao. 1983; 6:978–980.
6. Xu RN. Parapapillary choledochoduodenal fistula: diagnosis and treatment. Zhonghua Wai Ke Za Zhi. 1984; 22:672–673. 701
7. He SL, Tang RS. A case report of choledochoduodenal fistula. Lichuang Fangshexue Za Zhi. 1984; 3:120–121.
8. Jiao TY. A case report of choledochoduodenal fistula. Shanghai Med J. 1985; 5:420–421.
9. Dai XZ, Chen MZ. Clinical assessment of para-ampulla choledocho-duodenal fistulae in 27 cases. Zhonghua Nei Ke Za Zhi. 1986; 25:655–657. 700–701.
10. Li XC. Two case reports of choledochoduodenal fistula. Beijing Med J. 1987; 4:251–252.
11. Gu GH. Choledochoduodenal fistula: a long-term complication of T tuber drainage. J Third Mil Med Univ. 1987; 4:443.
12. Li Z. A case report of choledochoduodenal fistula after T tuber drainage. J Chin Pract Surg. 1991; 4:201.
13. Wang ZL, Lu Qide. A case report of choledochoduodenal fistula secondary penetrated duodenal peptic ulcer. J Pract Radiol. 1993; 3:507.
14. Fei X. Pedical A case report of omentum repairing the choledochoduodenal fistula. J Hepatobiliary Surg. 1995; 4:216.
15. Li YC. 2 Cases of choledochoduodenal fistula. Hainan Yi Xue. 1995; 2:135.
16. Zhu JR, Kai GW, Zhang YC. X-ray analysis for choledochoduodenal fistula. J Pract Radiol. 1995; 3:183–184.
17. Hu MX. 13 Cases of choledochoduodenal fistula after T tuber drainage. Curr Physician. 1996; 12:34.
18. Shen WS, Zhao P. 4 Cases of choledochoduodenal fistula. J Chin Pract Surg. 1996; 8:486–487.
19. Zhao DL, You L. Cardiac cancer implicated with choledochoduodenal fistula. Chin J Clin Gastroenterol. 1996; 2:82.
20. Li WM. Surgical treatment for choledochoduodenal fistula: 11 cases report. J Mod Oper Surg. 1997; 4:286.
21. Fan XJ, Geng XQ. 4 Rare cases of choledochoduodenal fistula. Yunnan Yi Yao. 1997; 5:398–399.
22. Li NF, Zhang YD, Liu S. 69 Cases of choledochoduodenal fistula: a retrospective study of ERCP. Chin J Endosc. 1999; 4:34–35.
23. Gong JP, Zhou YB, Han BL. Diagnosis and classification of choledochoduodenal fistula. Chin J Digest Endosc. 1999; 2:42–43.
24. Gong JP, Zhou YB, Han BL. Diagnosis and surgical management of choledochoduodenal fistula. J Third Mil Med Univ. 2000; 22:287–289.
25. Wang XX, Tao F. Choledochoduodenal fistula induced by the partially prolapsed T tube: two cases report. Zhejiang Pract Med. 2001; 6:57–58.
26. Tian ZL, Wu DX. A case report of choledochoduodenal fistula. J Hebei Med Uni. 2001; 22:106.
27. Li ZH, Zhou YB, Chen M, Liu JK, Chen K. Clinical analysis of juxtapapillary choledochoduodenal fistula: report of 47 cases. Chin J Gen Surg. 2001; 16:331–333.
28. Ku RX, Chen F, Liu SL, Wei NR, Ge XH, Ablet . Diagnosis of choledochoduodenal fistula by barium meal analysis: a case report. Xinjiang Med J. 2001; 1:65.
29. Xie J. A case report of choledochoduodenal fistula after T tuber drainage. J Clin Surg. 2002; 10:10.
30. Yu HZ, Li FY, Du Y, Geng XP, Xiong QR. Diagnosis and treatment of choledochoduodenal fistul. J Clin Surg. 2002; 10:88–89.
31. Gu J, Li JS, Ren J AN. Discussion of the suitable management and preventive methods for distal choledochoduodenal fistula. Chin J Gen Surg. 2002; 17:725–726.
32. Hou YL, Chen ZW, Cheng H, Fu G. Analysis of 7 cases of postoperative distal choleo-duodonum fistulas. Fu Bu Wai Ke. 2004; 17:230–231.
33. Hao RR, Yu L, Wang HJ. A case report of gallstone ileus induced by choledochoduodenal fistul. Beijing Med. 2005; 27:719.
34. Lan XH, Xu L, Wang LY, Wang YZ. Diagnosis and treatment for peripapillary choledo-choduodenal fistula. Clin Med Chin. 2005; 1:71–72.
35. Li ZH, Ding J, Ye Y, Cai L, Liu X, Liu J, et al. New strategy to prevent ascending cholangitis in larger choledochoduodenal fistula. ANZ J Surg. 2006; 76:796–800.
36. Zhao DP, Sun W, Xu HM, Ma YW. 7 Cases of choledochoduodenal fistula after T tuber drainage. Acta Acad Med Weifang. 2006; 28:76.
37. Lin D, Zhang LH, Jiang TR, Lan SQ, Yang LK. 16 Cases reports of choledochoduodenal fistula diagnosed by ERCP. Fujian Med J. 2006; 28:118–119.
38. Peng B, Du JP, Deng B, Cun BY, Jiang LL, Zhang SF. A case report of primary pleomorphic intrahepatic cholangiocarcinoma complicated with choledochoduodenal fistula. J Sichuan Univ. 2006; 3:455.
39. Sheng W, Zhao J, Luo HY, Liu ZB. A case report of choledochoduodenal fistula. J Clin Radiol. 2007; 26:87.
40. Chen HP, Bai L, Li WB, Liang C, Han SX. A case report of choledochoduodenal fistula induced by foreign bodies on the border of duodenum ball drop. Chin J Endosc. 2007; 13:1341–1342.
41. Xu ZY, Liu L, Zhang CQ, Li F, Li QQ. Clinic analysis 0f 9 cases with choledochoduodenal fistula associated with pancreatic carcinoma. Chin J Mod Med. 2007; 17:1386–1388. 1392
42. Wang An G. 5 Cases of choledochoduodenal fistula after T tuber drainage. J Liaoning Med Univ. 2010; 31:73–74.
43. Chen RB. A case report of choledochoduodenal fistula. J Yangtze Univ. 2010; 1:107–108.
44. Wang WZ, He CH, Yi HF. 2 Cases of spontaneous chronic choledochoduodenal fistula. J Pract Med. 2010; 26:4050.
45. Zong KC, You HB, Gong JP, Tu B. Diagnosis and management of choledochoduodenal fistula. Am Surg. 2011; 77:348–350.
46. Li SW, Tian CP, Feng MH. A case report of choledochoduodenal fistula complicated cholecystolithiasis and choledocholithiasis. Public Med Forum Mag. 2012; 34:4632.
47. Yi XL, Li XH, Wang J. Management of choledochoduodenal fistula. For All Health. 2012; 18:66–67.
48. Ding CL, Ma D. Management of choledochoduodenal fistula. Chin J Aesthet Med. 2012; 21:84–85.
49. Liu Q, Lu MX, Zhang TH, Feng CL. 78 Cases report of choledochoduodenal fistula. Chin J Gerontol. 2012; 32:3534–3535.
50. Li CS, Yuan D, Zhang J, Yuan YS. A case report of choledochoduodenal fistula diagnosed by ERCP. J Chin Physician. 2012; 14:718.
51. Zhao S, Wang J, Ge J, Zhang X, Liu J, Zhang A, et al. Implantation of covered selfexpandable metal stent in the common bile duct for the treatment of choledochoduodenal fistula. J Clin Gastroenterol. 2014; 48:383–384.
52. Zhang YL, Xu JG, Zhu YY. Analysis of diagnosis and surgical treatment of the choledochoduodenal fistula. Chin J Mod Med. 2014; 29:103–105.
53. Halabi WJ, Kang CY, Ketana N, Lafaro KJ, Nguyen VQ, Stamos MJ, et al. Surgery for gallstone ileus: a nationwide comparison of trends and outcomes. Ann Surg. 2014; 259:329–335.
54. Chang WT, Lin NC, Hsia CY, Liu CS, Tsai HL, Loong CC. Liver transplantation for a renal transplantation recipient with secondary sclerosing cholangitis by choledochoduodenal fistula. Asian J Surg. 2012; 35:49–52.
55. Neumann H, Nagel A, Bernatik T, Wickles N, Neurath MF, Raithel M. Endoscopic closure of large, spontaneous, choledochoduodenal fistula by using an overthe-scope clip. Gastrointest Endosc. 2011; 74:200–202.
56. Hong T, Xu XQ, He XD, Qu Q, Li BL, Zheng CJ. Choledochoduodenal fistula caused by migration of endoclip after laparoscopic cholecystectomy. World J Gastroenterol. 2014; 20:4827–4829.
57. Moghaddam JA, Amini M, Adibnejad S. Development of bile duct bezoars following cholecystectomy caused by choledochoduodenal fistula formation: a case report. BMC Gastroenterol. 2006; 6:1.
58. Williamson JB, Draganov PV. Choledochoduodenal fistula after biliary metallic stent placement for pancreatic cancer. Dig Endosc. 2014; 26:292.
59. Chaudhari D, Saleem A, Murthy R, Baron T, Young M. Choledochoduodenal fistula after biliary placement of a selfexpanding metallic stent for palliation of pancreatic cancer. Endoscopy. 2013; 45:Suppl 2 UCTN. E77.
60. Okabe Y, Kaji R, Ishida Y, Noda T, Sasaki Y, Tsuruta O, et al. Successful endoscopic extraction of a large impacted choledocholithiasis in the ampulla of vater: two interesting cases. Dig Endosc. 2010; 22:Suppl 1. S103–S106.
61. Song TJ, Hyun YS, Lee SS, Park do H, Seo DW, Lee SK, et al. Endoscopic ultrasoundguided choledochoduodenostomies with fully covered self-expandable metallic stents. World J Gastroenterol. 2012; 18:4435–4440.
62. Chikamori F, Okumiya K, Inoue A, Kuniyoshi N. Laparoscopic cholecystofistulectomy for preoperatively diagnosed cholecystoduodenal fistula. J Gastroenterol. 2001; 36:125–128.
63. Karincaoglu M, Yildirim B, Kantarceken B, Aladag M, Hilmioglu F. Association of peripapillary fistula with common bile duct stones and cholangitis. ANZ J Surg. 2003; 73:884–886.
64. Ikeda S, Okada Y. Classification of choledochoduodenal fistula diagnosed by duodenal fiberscopy and its etiological significance. Gastroenterology. 1975; 69:130–137.
65. Yang D, Wang Z, Duan ZJ, Jin S. Laparoscopic treatment of an upper gastrointestinal obstruction due to Bouveret's syndrome. World J Gastroenterol. 2013; 19:6943–6946.
66. Mohammed N, Godfrey EM, Subramanian V. Cholecysto-duodenal fistula as the source of upper gastrointestinal bleeding. Endoscopy. 2013; 45:Suppl 2 UCTN. E250–E251.
67. Miyamoto S, Furuse J, Maru Y, Tajiri H, Muto M, Yoshino M. Duodenal tuberculosis with a choledocho-duodenal fistula. J Gastroenterol Hepatol. 2001; 16:235–238.
68. Jaballah S, Sabri Y, Karim S. Choledochoduodenal fistula due to duodenal peptic ulcer. Dig Dis Sci. 2001; 46:2475–2479.
69. Mueller XM, Tevaearai HT, Stumpe F, Hurni M, Ruchat P, Fischer AP, et al. Gastrointestinal disease following heart transplantation. World J Surg. 1999; 23:650–655.
70. Povoski S, Shamma'a J. Fistula involving portal vein and duodenum at the site of a duodenal ulcer in a patient after previous extrahepatic bile duct resection and brachytherapy. Dig Surg. 2003; 20:53–55.
71. Choi D, Lim HK, Kim MJ, Kim SJ, Kim SH, Lee WJ, et al. Liver abscess after percutaneous radiofrequency ablation for hepatocellular carcinomas: frequency and risk factors. AJR Am J Roentgenol. 2005; 184:1860–1867.
72. Ohtsuka T, Tanaka M, Inoue K, Nabae T, Takahata S, Yokohata K, et al. Is peripapillary choledochoduodenal fistula an indication for endoscopic sphincterotomy? Gastrointest Endosc. 2001; 53:313–317.
73. Dwivedi AN, Kumar S, Rana S, Maurya B. Transmural invasion of hepatic flexure of colon causing cholecystocolic fistula by aggressive gallbladder carcinoma. World J Surg Oncol. 2013; 11:86.
74. Agarwal N, Sharma BC, Garg S, Kumar R, Sarin SK. Endoscopic management of postoperative bile leaks. Hepatobiliary Pancreat Dis Int. 2006; 5:273–277.
75. Periselneris N, Bong JJ. Choledocho-duodenal fistula encountered during emergency laparotomy for upper gastro-intestinal haemorrhage: what should be the surgical strategy? Clin Ter. 2011; 162:547–548.
76. Lee JH, Han HS, Min SK, Lee HK. Laparoscopic repair of various types of biliaryenteric fistula: three cases. Surg Endosc. 2004; 18:349.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download