Abstract
Purpose
One of the major causes of bowel obstruction in extremely premature infants is a meconium obstruction. However, there are many challenges not only in the recognition and diagnosis, but also in the management of meconium obstruction. This study aimed to find perioperative clinical features and determine the postoperative course of meconium-related ileus in very low birth weight (VLBW) and extremely low birth weight (ELBW) infants.
Methods
We retrospectively reviewed the clinical data of premature infants (n = 11, VLBW infnats; n = 16, ELBW infants) with a meconium-related ileus who underwent operation for intractable ileus between January 2009 and May 2013.
Results
The average duration of conservative management was longer and postnatal age was older in ELBW infants than VLBW infants: 19.9 days vs. 11.5 days and 34.9 days vs. 19.2 days. The immediate postoperative course (day that beginning feeding and full feeding) was not significantly different based on birth weight, but the ELBW infants had slightly higher mortality. At 12 months of corrected age after operation, both average body weight and average height was below 10th percentile for growth in most infants (61.1%).
Conclusion
There was a slightly high mortality in the ELBW infants, but two groups did not experience significant differences in the immediate postoperative course of meconium-related ileus. Nevertheless, considering their growth patterns, it is necessary to do a close follow-up and more aggressive nutritional management to achieve optimal growth and development in both patient groups.
Intestinal obstruction in the newborn infants is associated with a variety of conditions, including meconium obstruction which can presents in extremely premature infants with very low birth weight (VLBW) or extremely low birth weight (ELBW). It poses a significant challenge not only for recognition and diagnosis, but also for the management. With the increased survival and live-birth rates of VLBW and ELBW infants, the incidence of meconium obstruction could also increase [12].
Intestinal obstruction due to meconium in premature infants was previously considered to be closely related to cystic fibrosis (CF), however, many cases without CF have been reported after the first description of meconium obstruction in 1965 [34]. It usually presents as an intestinal obstruction caused by an inspissated meconium in the terminal ileum [235].
This condition can be resolved by conservative management; however, surgical intervention may be needed in some cases with an intractable ileus. Delayed diagnosis and management of it might result in increased morbidity and mortality in these extremely premature infants.
This study aimed to investigate the perioperative clinical features and determine the postoperative course of VLBW and ELBW infants who underwent an operation for a meconium-related ileus (MRI), with particular emphasis on the immediate and 1-year postoperative status.
We assessed all premature infants managed for MRI between January 2009 and May 2013, resulting in 11 VLBW and 16 ELBW infants who underwent surgery for intractable ileus and were included after written informed consent was obtained from the parent.
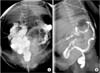
These patients satisfied the following criteria: (1) clinical presentation with reduced frequency of defecation as well as abdominal distention and bilious or nonbilious vomiting and (2) radiologic findings of distended small bowel loops without air-fluid levels or pneumatosis on abdominal plain radiography (Fig. 1) and a normal-sized colon or microcolon with multiple filling defects on contrast enemas (Fig. 2) [6789].
Gestational age, birth weight, cause of prematurity, duration of attempted conservative management prior to surgery, postnatal age at the time of surgery, and associated conditions were reviewed for all subjects.
The decision for surgical managements was based on clinical deterioration despite conservative care and to exclude other pathologic conditions (e.g., Hirschsprung disease, intestinal neuronal dysplasia, or hypoganglionosis of the colon). An appendectomy was performed for histologic examination and followed by a loop enterostomy after bowel irrigation and evacuation of the meconium through a small enterotomy at the site of the transitional zone formed by the impacted meconium. The inspissated meconium was usually located within 20 cm from the ileocecal junction (Figs. 3, 4).
During the postoperative course, mortality was noted in eight infants (n = 3, VLBW; n = 5, ELBW), and 1 VLBW infant was lost follow-up. These cases were excluded from the assessment of postoperative progress. Immediate postoperative clinical course was assessed using the following parameters: (1) day that feeding began, (2) day of full feeding (full feeding was defined as at least 120 mL/kg/day), and (3) the duration until and postmenstrual age (PMA) at the time of enterostomy repair. The enterostomy repair was considered after infants had adapted to at least 120 mL/kg/day bottle-feeding, experienced weight gain, and no delayed passage of material after contrast enema. Body weight and height at 12 months of corrected age were assessed at follow-up.
The average gestational ages were 28.5 weeks (range, 26-35 weeks) and 26.2 weeks (range, 24-30 weeks), and the average birth weights were 1,147 g (range, 1,030-1,310 g) and 798 g (range, 460-990 g) for the VLBW and ELBW infants, respectively. The most common cause of premature birth was preterm labor following a premature rupture of membrane in VLBW infants and spontaneous preterm labor in ELBW infants (Table 1).
Conservative management was attempted in all cases until surgery, with average durations of 11.5 days for VLBW infants and 19.9 days for ELBW infants. The average postnatal age at the time of surgery was 19.2 and 34.9 days for the VLBW and ELBW infants, respectively. Associated conditions included a respiratory distress syndrome in all patients, and a patent ductus arteriosus and intracranial hemorrhage were also common in both VLBW and ELBW infants. In the postoperative course, a mortality occurred in 27.2% of VLBW infants and 31.3% of ELBW infants; all of these infants experienced a clinical deterioration following a septic condition despite surgical management (Table 2).
Enteral feeding was started at an average of 8.9 and 9.4 days after surgery, and the average duration to full-feeding (120 mL/kg/day) was 33.1 and 38.3 days for the VLBW and ELBW infants, respectively. The average duration until repair of the loopenterostomy was 50.3 and 83.7 days after initial surgery, at an estimated average PMA of 39.3 and 46.2 weeks for the VLBW and ELBW infants, respectively. There were no significant differences between VLBW and ELBW infants in the clinical findings in the immediate postoperative period (Table 3).
The growth patterns at a corrected age of 12 months for all survivors are shown in Table 4 and Figs. 5, 6. The average body weight was 8.4 kg and 7.2 kg, and the average height was 72.0 cm and 68.7 cm in the VLBW and ELBW infants, respectively. However, the percentile of growth pattern was <10th percentile in the majority of infants (61.1%).
Several types of meconium obstruction have been described since the first report by Clatworthy Jr et al. [10] in 1956. Although this obstruction usually occurs in premature newborns, there are no established diagnostic criteria, and the incidence has been increasing owing to better survival and live births of extremely premature neonates with better medical care over the past 2 decades [611]. However, the term MRI has described both meconium plug syndrome (MPS) and meconium disease [310]. MPS is a functional obstruction in the small intestine or proximal right colon caused by impaired meconium excretion. It appears to be associated with immature intestinal function and dysmotility in premature infants, with a multifactorial etiology. Milla [12] described that a more mature migrating motor complex of the intestine was evident only after 34-35 weeks of gestation, while Yoo et al. [13] proposed that a delayed maturity of interstitial cells of Cajal could cause meconium obstruction. Some reports have also described this condition as an immaturity of the myenteric nervous system or morphological immaturity of ganglia [1415]. Our study excluded these etiologies through histological examination, which revealed the presence of mature normal ganglion in the submucous and myenteric plexus. Other studies have suggested an association between MRI and the following maternal factors: a maternal diabetes, a preeclampsia or eclampsia, and chronic hypertension [16]. In the present study, preterm labor caused by a spontaneous or premature rupture of membrane was the most common cause of prematurity. Therefore, further studies should be conducted regarding a potential association between maternal factors and MRI. Although the varying associated conditions in the VLBW and ELBW infants did not appear to directly affect MRI, the clinical course of MRI could be influenced by these conditions.
Management of meconium obstruction in VLBW and ELBW infants is often challenging. It is difficult to clinically distinguish MRI from other causes of bowel obstructions including distal ileal atresia, ileal volvulus, and long segment Hirschsprung disease. Conservative methods of management include rectal stimulation with N-acetylcysteine (NAC), glycerin, or contrast enemas and enteral administration of Gastrografin or NAC via a nasogastric tube [917181920]. Previous studies have suggested that conservative management within 10 days of symptom development could be effective, and a delay in the initiation of therapy could increase the chance of requiring surgical intervention [202122]. The ELBW infants underwent surgery at a later postnatal age due to uncorrected thrombocytopenia and acidosis, intractable ileus, and anticipation of more surgical complications in the ELBW than in the VLBW infants. Contrary to early mortality rates of 50% to 67%, a survival rate of near or at 100% have been reported in meconium ileus over the past three decades [2324]. In this study, the mortality rate in the postoperative course was higher in the ELBW than in the VLBW infants (31.3% vs. 27.2%), but it was not significantly correlated with birth weight (P = 0.824). The higher postoperative morbidity and mortality is attributable to the fragile intestines and vulnerability of the hepatic capsule to tearing on manipulation. Hence, surgery is preferably avoided in ELBW infants [20].
Feeding patterns after surgical procedure-beginning enteral feeding and time to full-feeding-were not significantly different based on birth weight in the immediate postoperative period. None of the infants developed necrotizing enterocolitis after feeding; there were also no recurrent MRI after surgery. This suggests that most MRI cases are not progressive and that feeding can be started as early as tolerable in VLBW and ELBW infants. The mean duration until enterostomy repair was between 7 and 12 weeks in both groups, and there was no difference in the clinical course after the repair. Despite the lack of standard guidelines on the optimal timing of enterostomy repair, the timing in infants with necrtotizing enterocolitis reportedly varies from as early as 8 weeks to as late 10 weeks [2526]. In our study, the duration until repair was shorter in the VLBW than in the ELBW infants. However, when corrected for PMA, it was an average of 39.3 and 46.2 weeks in the VLBW and ELBW infants, respectively. Therefore, repair can be performed after a PMA of 40 weeks even though they are extremely premature infants.
Mean body weight and height at 12 months of corrected age was below the 10th percentile in VLBW infants and below the 3rd percentile in ELBW infants. In our study, catch-up growth (above the 10th percentile) at 12 months of corrected age was observed in only four VLBW infants (57.1%) and three ELBW infants (27.3%). It has been consistently shown that early pre-terms have a tendency to experience growth restraint [2728]. Most infants born before 30 weeks do not attain the median birth weight of a reference fetus with the same gestational age at the time of discharge [2930]. VLBW and ELBW infants could experience postnatal catch-up growth until 3 years of age, but mainly in the 1st year [29]. Studies have reported that 89% of ELBW infants have a growth pattern below the 10th percentile at the time of discharge, and only 60% attained a catch-up growth at 12 months of corrected age [30]. Our study demonstrates that ELBW infants receiving surgical management for MRI achieved growth pattern values that are lower than those previously reported. Considering the failure to thrive in these infants, it is necessary to closely monitor growth and nutritional status in the first year of life. It is also essential to provide additional calories to optimize the growth and minimize the failure to thrive, especially in the first year of life, in VLBW and ELBW infants who underwent operation for MRI.
Our study is limited by the small sample size and retrospective study design in single center. Prospective and Larger, multicenter studies are required to identify a comprehensive protocol for the management of MRI and provision of efficient nutritional support during early life.
In conclusion, although the mortality was higher in VLBW and ELBW infants, they showed not so different clinical course in the immediate postoperative period. Considering the high postoperative morbidity and mortality, it is necessary to properly plan the medical and surgical management based on individual clinical parameters. Furthermore, anticipated poor growth in these infants warrants close follow-up and more aggressive nutritional management for optimal growth during the first year of life.
Figures and Tables
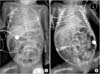 | Fig. 1Abdominal radiography shows gaseous distention of the small bowel loop without air-fluid level in A, B. |
 | Fig. 3Operative finding also shows a gaseous distention of small intestinal loop and collapsed distal ileum filled with sticky meconium causing obstruction. |
 | Fig. 4Sticky, mucoid meconium removed from intestine and an impacted meconium looking like a hard, thick strand in inlet. |
 | Fig. 5The percentile of growth: body weight at corrected age of 12 months. VLBW, very low birth weight; ELBW, extremely low birth weight. |
 | Fig. 6The percentile of growth: height at corrected age of 12 months. VLBW, very low birth weight; ELBW, extremely low birth weight. |
References
1. Greenholz SK, Perez C, Wesley JR, Marr CC. Meconium obstruction in markedly premature infant. J Pediatr Surg. 1996; 31:117–120.
2. Kubota A, Imura K, Yagi M, Kawahara H, Mushiake S, Nakayama M, et al. Functional ileus in neonates: Hirschsprung's disease-allied disorders versus meconium-related ileus. Eur J Pediatr Surg. 1999; 9:392–395.
3. Rickham PP, Boeckman CR. Neonatal meconium obstruction in the absence of mucoviscidosis. Am J Surg. 1965; 109:173–177.
4. Siegel MJ, Shackelford GD, McAlister WH. Neonatal meconium blockage in the ileum and proximal colon. Radiology. 1979; 132:79–82.
5. Hatanaka A, Nakahara S, Takeyama E, Iwanaka T, Ishida K. Management of extremely low birth weight neonates with bowel obstruction within 2 weeks after birth. Surg Today. 2014; 44:2269–2274.
6. Dimmitt RA, Moss RL. Meconium diseases in infants with very low birth weight. Semin Pediatr Surg. 2000; 9:79–83.
7. Karimi A, Gorter RR, Sleeboom C, Kneepkens CM, Heij HA. Issues in the management of simple and complex meconium ileus. Pediatr Surg Int. 2011; 27:963–968.
8. Kubota A, Shiraishi J, Kawahara H, Okuyama H, Yoneda A, Nakai H, et al. Meconium-related ileus in extremely low-birthweight neonates: etiological considerations from histology and radiology. Pediatr Int. 2011; 53:887–891.
9. Emil S, Nguyen T, Sills J, Padilla G. Meconium obstruction in extremely low-birth-weight neonates: guidelines for diagnosis and management. J Pediatr Surg. 2004; 39:731–737.
10. Clatworthy HW Jr, Howard WH, Lloyd J. The meconium plug syndrome. Surgery. 1956; 39:131–142.
11. Coppola CP. Meconium plug syndrome and meconium ileus. In : Coppola CP, Kennedy AP, Scorpio RJ, editors. Pediatric surgery: diagnosis and treatment. Cham (CH): Springer International Publishing;2014. p. 183–185.
12. Milla PJ. Development of intestinal structure and function in neonatal gastroenterology. In : Tanner MS, Stocks RJ, editors. Neonatal gastroenterology: contemporary issues. Newcastle upon Tyne(UK): Intercept;1984. p. 1–20.
13. Yoo SY, Jung SH, Eom M, Kim IH, Han A. Delayed maturation of interstitial cells of Cajal in meconium obstruction. J Pediatr Surg. 2002; 37:1758–1761.
14. Toyosaka A, Tomimoto Y, Nose K, Seki Y, Okamoto E. Immaturity of the myenteric plexus is the aetiology of meconium ileus without mucoviscidosis: a histopathologic study. Clin Auton Res. 1994; 4:175–184.
15. Bughaighis AG, Emergy JL. Functional obstruction of the intestine due to neurological immaturity. Prog Pediatr Surg. 1971; 3:37–52.
16. Ziegler MM. Meconium ileus. Curr Probl Surg. 1994; 31:731–777.
17. Shaw A. Safety of N-acetylcysteine in treatment of meconium obstruction of the newborn. J Pediatr Surg. 1969; 4:119–125.
18. Noblett HR. Treatment of uncomplicated meconium ileus by Gastrografin enema: a preliminary report. J Pediatr Surg. 1969; 4:190–197.
19. Shinohara T, Tsuda M, Koyama N. Management of meconium-related ileus in very low-birthweight infants. Pediatr Int. 2007; 49:641–644.
20. Garza-Cox S, Keeney SE, Angel CA, Thompson LL, Swischuk LE. Meconium obstruction in the very low birth weight premature infant. Pediatrics. 2004; 114:285–290.
21. Paradiso VF, Briganti V, Oriolo L, Coletta R, Calisti A. Meconium obstruction in absence of cystic fibrosis in low birth weight infants: an emerging challenge from increasing survival. Ital J Pediatr. 2011; 37:55.
22. Chan KL, Ng SP, Chan KW, Wo YH, Tam PK. Pathogenesis of neonatal necrotizing enterocolitis: a study of the role of intraluminal pressure, age and bacterial concentration. Pediatr Surg Int. 2003; 19:573–577.
23. Carlyle BE, Borowitz DS, Glick PL. A review of pathophysiology and management of fetuses and neonates with meconium ileus for the pediatric surgeon. J Pediatr Surg. 2012; 47:772–781.
24. Del Pin CA, Czyrko C, Ziegler MM, Scanlin TF, Bishop HC. Management and survival of meconium ileus. A 30-year review. Ann Surg. 1992; 215:179–185.
25. Al-Hudhaif J, Phillips S, Gholum S, Puligandla PP, Flageole H. The timing of enterostomy reversal after necrotizing enterocolitis. J Pediatr Surg. 2009; 44:924–927.
26. Struijs MC, Sloots CE, Hop WC, Tibboel D, Wijnen RM. The timing of ostomy closure in infants with necrotizing enterocolitis: a systematic review. Pediatr Surg Int. 2012; 28:667–672.
27. Hack M, Schluchter M, Cartar L, Rahman M, Cuttler L, Borawski E. Growth of very low birth weight infants to age 20 years. Pediatrics. 2003; 112(1 Pt 1):e30–e38.
28. Cooke RJ, Ainsworth SB, Fenton AC. Postnatal growth retardation: a universal problem in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2004; 89:F428–F430.
29. Kytnarova J, Zlatohlavkova B, Kubena A, Markova D, Dokoupilova M, Plavka R, et al. Post-natal growth of 157 children born as extremely premature neonates. J Paediatr Child Health. 2011; 47:111–116.
30. Steward DK, Pridham KF. Growth patterns of extremely low-birth-weight hospitalized preterm infants. J Obstet Gynecol Neonatal Nurs. 2002; 31:57–65.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download