Abstract
Spontaneous coronary artery dissection (SCAD) is a very rare cause of peripheral artery thromboembolism. It is especially rare to show symptoms of acute limb ischemia without chest symptoms during a hospital visit. In this case, a rare case of SCAD led to left heart failure and caused left ventricle thrombi, which in turn caused peripheral thromboembolism.
Acute limb ischemia (ALI) presents acute symptoms of leg pain due to thromboembolism, mostly. For the aortoiliac artery, in particular, large thromboemboli that arise from a cardiac or other proximal source and lodge at the aortic bifurcation can lead to profound acute lower extremity ischemia [1]. Thromboembolism from a cardiac source is mostly caused by atrial fibrillation [2], and rarely by spontaneous coronary artery dissection (SCAD).
Acute coronary syndrome is rarely caused by SCAD and the incidence of SCAD was reported in 0.1%-1.1% [3]. SCAD shows various clinical presentations such as myocardial infarction, cardiogenic shock, and sudden death, and often presents similar symptoms with atherosclerotic coronary artery disease, which makes it difficult to make an accurate diagnosis [4].
A 35-year-old man with no known prior medical history presented with right leg pain with stable vital signs. The right leg pain continued from a week prior and chest pain was extreme a week before, but showed improved state on arrival at the hospital. The right leg showed resting pain and coldness, but motor and sensory activities were intact and viable.
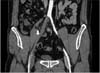
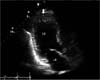
In CT angiography, total thrombotic occlusion was revealed from right common iliac artery to superficial femoral artery (Fig. 1). An electrocardiogram demonstrated anterolateral myocardial infarction. Transthoracic echocardiogram demonstrated several thrombi at left ventricle apex and akinesia from left ventricle apex to apical anterior wall (Fig. 2) but ejection fraction was maintained at 55%. The largest thrombi was measured at 17.4 mm.
Cardiac enzymes showed normal results of troponin I and creatine kinase-myoglobin and blood tests identified no abnormal findings. For hypercoagulability study, laboratory results of homocystein, antiphospholipid antibody, antithrombin III, protein C activity and factor V leiden mutation were normal, but protein S activity was slightly decreased at 59% (normal > 77%). Drug screen tests of Cocaine, Cannabinoids and Morphine were negative.
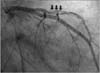
From arrival at our institution, heparin was infused continuously. After contacting the Cardiology Department, coronary angiography was performed, which demonstrated dissection of left anterior descending (LAD) coronary artery (Fig. 3).
Currently, left ventricle thrombi do not induce severe heart failure, and coronary artery dissection is vulnerable and distal flow is maintained. Therefore, conservative treatment is maintained using heparin, and intervention will take place when dissection is stabilized.
Therefore, thrombectomy was performed on the right ileofemoral thromboembolism under general anesthesia. First, through the right femoral artery of the inguinal, proximal and distal thrombectomy was done with Fogarty balloon catheter and for the tibial lesions of thrombosis, diagnostic angiography and aspiration thrombectomy were done with an interventional radiologist. Echocardiogram was done pre- and postoperatively with no medical events postoperatively.
After operation, aspirin and warfarin were taken. After postoperative week 2, follow-up echocardiography demonstrated decreased size of left ventricle thrombi and reserved left ventricle function. The durable fluoropolymer-based everolimus-eluting stent (Xience Prime, Promus, Boston Scientific, Natick, MA, USA) insertion for coronary artery dissection of LAD was done. In follow-up CT angiography, ileofemoral thromboemboli disappeared and distal flows were recovered with scanty thrombi.
There is a significant association between SCAD and pregnancy. This is due to hormonal variations that influence the composition of the vessel [5]. Other conditions that have been associated with dissections are: systemic lupus erythematosus, blunt thoracic trauma, sarcoidosis, fibromuscular dysplasia, cardiopulmonary resuscitation, and use of cocaine, among others [5,6]. Predisposing factors include severe hypertension, smoking, collagen disorders and oral contraception [6].
The patient was in good health, but after a stressful event a week before coming to the hospital, complained of temporary chest pain. The pain improved but afterward presented with ALI.
It is very difficult to suspect the diagnosis of SCAD during acute coronary syndrome, because the incidence is very low (0.1%-1%) and patient symptoms and clinical presentation are similar to atherosclerotic coronary artery disease [3].
For the mechanism of SCAD, an intimal tear may lead to separation of coronary wall layers and, eventually, to a double lumen. Pressure-driven expansion of the false lumen induces axial propagation of the disease and true lumen compression, resulting in myocardial ischemia and heart failure [7].
For the aortoiliac artery, large thromboemboli that arise from a cardiac or other proximal source and lodge at the aortic bifurcation, referred to as saddle emboli, can lead to profound acute lower extremity ischemia [1]. Such ALI can cause cardiac arrhythmia, endocarditis, cardiac tumor, atheroembolism, aortic mural thrombi, etc. [8].
In this case, a rare case of SCAD led to left heart failure and caused left ventricle thrombi, which in turn caused peripheral thromboembolism. There are a few reports on pulmonary thromboembolism due to SCAD [9], however peripheral artery occlusive disease due to SCAD are rarely found.
In the treatment of SCAD, if heart failure is not severe, percutaneous coronary intervention (PCI) is not performed during the acute stage. PCI is associated with high risk for technical failure due to wire passage into the false lumen and propagation of dissection [10]. Therefore, in the acute stage, anticoagulation was done, and PCI was performed in a stable condition.
In conclusion, SCAD is a very rare cause of peripheral artery thromboembolism. It is especially rare to show symptoms of ALI without chest symptoms during a hospital visit. If a young, healthy person with no special history of medical illness shows symptoms of ALI, heart evaluation must be done and SCAD should also be considered.
Figures and Tables
References
1. Wingo JP, Nix ML, Greenfield LJ, Barnes RW. The blue toe syndrome: hemodynamics and therapeutic correlates of outcome. J Vasc Surg. 1986; 3:475–480.
2. Inoue H, Atarashi H. Research Group for Antiarrhythmic Drug Therapy. Risk factors for thromboembolism in patients with paroxysmal atrial fibrillation. Am J Cardiol. 2000; 86:852–855.
3. Hering D, Piper C, Hohmann C, Schultheiss HP, Horstkotte D. Prospective study of the incidence, pathogenesis and therapy of spontaneous, by coronary angiography diagnosed coronary artery dissection. Z Kardiol. 1998; 87:961–970.
4. Dhawan R, Singh G, Fesniak H. Spontaneous coronary artery dissection: the clinical spectrum. Angiology. 2002; 53:89–93.
5. Hinojal YC, Di Stefano S, Florez S, Martinez G, de la Fuente L, Casquero E, et al. Spontaneous coronary dissection during postpartum: etiology and controversies in management. Ital Heart J. 2004; 5:563–565.
6. Almeda FQ, Barkatullah S, Kavinsky CJ. Spontaneous coronary artery dissection. Clin Cardiol. 2004; 27:377–380.
7. Vrints CJ. Spontaneous coronary artery dissection. Heart. 2010; 96:801–808.
8. Swartz MF, Lutz CJ, Chandan VS, Landas S, Fink GW. Atrial myxomas: pathologic types, tumor location, and presenting symptoms. J Card Surg. 2006; 21:435–440.
9. Gul I, Basar E, Cetinkaya Y, Kasapkara A, Kalay N, Ozdogru I. Spontaneous coronary artery dissection and pulmonary thromboembolism: a case report. Int J Cardiol. 2007; 118:e21–e23.
10. Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, Lennon RJ, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. 2012; 126:579–588.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download