Abstract
We report a rare case of sepsis with acute respiratory distress syndrome (ARDS) caused by Candida parapsilosis and Candida famata after a small bowel bezoar operation. The patient was successfully treated with intensive care including mechanical ventilation and systemic antifungal therapy. A strong association was observed between the intestinal obstruction caused by the bezoar and candidemia presenting as ARDS. This is the first case in which candidemia has led to ARDS after a bezoar removal operation in a patient who was neither immunocompromised nor self-administering an illicit intravenous drug.
Candida has become the fourth most common pathogen of nosocomial blood stream infections, which represents a substantial increase [1]. The source of these pathogens has been a subject of considerable debate, with suggestions divided between the gastrointestinal tract (endogenous source) and the skin (exogenous source) [2]. Fungal bezoars or fungus balls which result in obstruction of the urinary tract have been reported as a source of candidemia [3]. However, intestinal obstruction from a bezoar has not been reported previously in association with candidemia. Here, we report a case of candidemia with acute respiratory distress syndrome (ARDS) caused by Candida parapsilosis and Candida famata in a patient after bezoar removal operation.
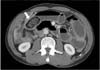
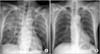
A 51-year-old man presented with abdominal pain and vomiting and was admitted for a suspected diagnosis of an intestinal obstruction. Several days prior to symptom onset, the patient had eaten uncertain quantities of persimmon. His medical history was significant for a previous abdominal operation to repair a small bowel perforation 20 years ago. The admission laboratory studies were significant for leukocytosis (19,240/µL) and a hemoglobin concentration of 18.3 g/d, but normal for C-reactive protein (0.07 mg/dL). Body temperature was 36.5℃. Abdominal distension was observed with mild tenderness over the epigastric area was noted. A serologic investigation for the immunocompromised state (HIV antibody) was negative. An abdominal CT scan revealed a mechanical obstruction in the ileum with a 5.5 × 2.5-cm ovoid bezoar and mild splenomegaly (Fig. 1). Laboratory studies at 4 days after admission were significant for decrease of leukocytosis (7,690/µL), but increase for C-reactive protein (4.02 mg/dL). Because his symptoms had not resolved by day 5 of hospitalization despite spontaneous intermittent decompression of small bowel obstruction, the patient underwent a bezoar removal operation. An intraoperative examination revealed small bowel dilatation with no signs of small bowel ischemia. The bezoar was identified in the ileum and was extracted through an enterotomy, followed by decompression of the dilated bowel. The bezoar was a 6.0 × 3.5 × 3.0 cm, green and sclerous (Fig. 2). The patient tolerated the procedure well. However, the postoperative course was complicated by recurrent episodes of fever (>38℃) on day 2, which was thought to be caused by postoperative atelectasis. The patient began to pass flatus and started on a progressive diet by postoperative day 5. On postoperative day 7, the patient developed dyspnea, and chest radiography showed diffuse ground glass opacity. Subsequent, ARDS was diagnosed, based on the PO2:FIO2 ratio and diffuse, bilateral radiographic consolidation (Fig. 3A). The patient was transferred to the intensive care unit and was supported on mechanical ventilation. Blood cultures collected from postoperative day 4 revealed yeast, and the patient was started on an empiric therapy of 400-mg intravenous fluconazole (Oneflu, Choongwae Pharma, Seoul, Korea) daily pending identification of the yeast. The blood cultures from postoperative day 4 eventually grew C. famata, and those from postoperative days 6 and 7 grew C. parapsilosis. Despite 4 days of intravenous fluconazole treatment, the fever and leukocytosis persisted, and an additional history of antifungal treatment for tinea pedis 3 years ago was obtained from family members. Therefore, the empiric therapy was changed to intravenous 325-mg amphotericin B (Ambisome, Yuhan Pharma, Seoul, Korea) daily. Additionally, intravenous 125-mg methylprednisolone (Salon, Hanlym Pharma, Seoul, Korea) was administrated daily for 14 days, followed by a tapering over a 2-week period. The stool culture collected from postoperative day 12 grew Candida glabrata. On postoperative day 18, the antifungal treatment regimen was changed to 50-mg intravenous caspofugin (Cancidas, MSD Sharp & Dohme GmbH, Schweiz, Germany) daily, because of amphotericin-associated nephrotoxicity. On day 7 of antifungal treatment, the patient became afebrile and showed clinical improvement (Fig. 3B). Blood cultures from days 7, 10 and 14 after the initiation antifungal therapy were sterile. In total, the patient had undergone a 30-day course of antifungal therapy (4 days of fluconazole, 7 days of amphotericin B, and 19 days of caspofugin) and was discharged on postoperative day 37.
The major sources of candidemia are the gastrointestinal tract and the skin [2]. The main factors for candidemia pathogenesis are breakdown of normal mucosal or skin barriers and loss of immune control mechanisms. Therefore, candidemia can be caused by abdominal surgery, mucosal damage from anticancer therapy, use of indwelling catheters, or immunosuppression. Bezoars in extragastrointestinal systems such as the urinary tract have been known as a source of candidemia. Most candidemia cases above have been reported in situations in which a urinary tract obstruction occurred due to fungal bezoars or fungus balls [3]. However, no reports are available on candidemia in association with an intestinal bezoar. No urinary or central venous catheters were used in our case until after the initial episode of postoperative fever, suggesting that the skin or urinary tract was unlikely sources for the candidemia. Therefore, we could presume a bezoar with intestinal obstruction as the cause of candidemia, despite the lack of reports on a definite relationship between intestinal bezoar and fungus.
Bezoars most commonly develop in the stomach, and small bowel bezoars are rare. Most bezoars in the stomach are treated by endoscopic destruction, whereas a surgical intervention is usually required for bezoar-induced small bowel obstructions, despite reports of small bowel bezoars passing spontaneously [4]. In our case, conservative management was selected due to the resolving symptoms of the small bowel obstruction for several days. However, an operation should be performed in a setting with a clinically deteriorating patient despite conservative management. Additionally, the continued unexplained fever and elevated white blood cell count in the setting of appropriate empiric antibacterial therapy after the abdominal operation for a bezoar with intestinal obstruction may be cardinal sign of candidemia, if other surgical complications can be ruled out. Thus, candidemia should always be considered in a work up of unexplained fever.
A marked increase in blood-stream infections due to candidemia from Candida species other than C. albicans has been observed. C. parapsilosis has been reported in the setting of sepsis and pulmonary dysfunction in immunocompromised patients [5]. However, no report has been published on candidemia and ARDS in a patient who is neither immunocompromised nor self-administering illicit intravenous drugs. Although in vitro susceptibility to C. famata by antifungal agents is lacking, C. famata appears to exhibit reduced susceptibility to echinocandins and azoles, and prompt initiation of therapy with liposomal amphotericin B is recommended for successful treatment, particularly for immunocompromised patients [6].
In conclusion, a small bowel bezoar with an intestinal obstruction could cause candidemia. Candidemia should always be considered in any patient with a persistent unexplained fever after an operation, including any condition that results in mucosal breakdown.
Figures and Tables
References
1. Pfaller MA, Diekema DJ, Jones RN, Sader HS, Fluit AC, Hollis RJ, et al. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and in vitro susceptibilities to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY antimicrobial surveillance program. J Clin Microbiol. 2001; 39:3254–3259.
2. Nucci M, Anaissie E. Revisiting the source of candidemia: skin or gut? Clin Infect Dis. 2001; 33:1959–1967.
3. Yoo SY, Namkoong MK. Acute renal failure caused by fungal bezoar: a late complication of Candida sepsis associated with central catheterization. J Pediatr Surg. 1995; 30:1600–1602.
4. Swift RI, Wood CB, Hershman MJ. Small bowel obstruction due to phytobezoars in the intact gastrointestinal tract. J R Coll Surg Edinb. 1989; 34:267–269.
5. Salo J, Ribera JM, Blade J, Puig de la Bellacasa J, Nomdedeu B, Granena A, et al. Sepsis caused by Candida parapsilosis. Joint and lung involvement in 2 patients with acute leukemia. Med Clin (Barc). 1990; 94:58–60.
6. Beyda ND, Chuang SH, Alam MJ, Shah DN, Ng TM, McCaskey L, et al. Treatment of Candida famata bloodstream infections: case series and review of the literature. J Antimicrob Chemother. 2013; 68:438–443.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download