Abstract
Purpose
The purpose of this study is to report the results of simultaneous pancreas-kidney (SPK) transplantations and describe the lessons learned from the early experiences of a single center.
Methods
Between January 2002 and June 2013, a total of 8 patients underwent SPK transplantation. Clinical and radiologic data were reviewed retrospectively.
Results
Seven patients were diagnosed with type I diabetes mellitus and one patient became insulin-dependent after undergoing a total pancreatectomy because of trauma. Pancreas exocrine drainage was performed by enteric drainage in 4 patients and bladder drainage in 4 patients. Three patients required conversion from initial bladder drainage to enteric drainage due to urinary symptoms and duodenal leakage. Four patients required a relaparotomy due to hemorrhage, ureteral stricture, duodenal leakage, and venous thrombosis. There was no kidney graft loss, and 2 patients had pancreas graft loss because of venous thrombosis and new onset of type II diabetes mellitus. With a median follow-up of 76 months (range, 2-147 months), the death-censored graft survival rates for the pancreas were 85.7% at 1, 3, and 5 years and 42.9% at 10 years. The patient survival rate was 87.5% at 1, 3, 5, and 10 years.
Conclusion
The long-term grafts and patient survival in the current series are comparable to previous studies. A successful pancreas transplant program can be established in a single small-volume institute. A meticulous surgical technique and early anticoagulation therapy are required for further improvement in the outcomes.
Simultaneous pancreas-kidney (SPK) transplantation is an established therapeutic approach for patients with insulin-dependent diabetes mellitus and end-stage renal disease [1]. Improvement of quality of life and potential reductions in macrovascular and microvascular complications have been documented in many publications [2,3,4]. In addition, SPK transplants have been reported to be superior in graft survival compared to kidney-only or solitary pancreas transplantation [5,6]. Despite the long-term benefits of SPK transplants, there are a lot of potential complications in the early postoperative period. Technical factors influence the early outcomes greatly. In spite of recent improvements in surgical technique and perioperative management, SPK transplantation is still thought to be associated with significant initial morbidity, and the graft failure rate remains also high [3,7]. Management of various morbidities in the early postoperative period requires the multidisciplinary approach. It has been believed to be too difficult to perform and maintain a pancreas transplantation program in a small-volume center because of these problems.
We performed our first SPK transplantation in 2002 and became the fourth institute performing SPK transplantations in Korea. We have maintained the transplantation program including SPK transplants and performed a total of 8 cases over 10 years. The purpose of this study was to report the results of the SPK transplantations and describe the lessons learned from early experiences of a small-volume center.
A total of 251 patients underwent solid organ transplantation, including kidney or liver transplantation, between January 2002 and June 2013. Of the 251 patients, 8 patients underwent simultaneous pancreas kidney transplantation. All the cases were performed by one transplantation surgeon. A retrospective review of the medical records and radiologic studies of these patients were done after approval by the Hallym University Kandong Sacred Heart Hospital Institutional Review Board (14-138)
The back-table procedure was done with standard methods using the donor iliac artery Y-graft. Implantations of both the pancreas and kidney were done intraperitoneally, except for one case in which the kidney was implanted retroperitoneally. The pancreas grafts were implanted on the right side of patients, by anastomosing the Y graft to the recipients' external iliac artery and the pancreas portal vein to the recipients' external iliac vein. Kidneys were implanted on the left side of patients with anastomosis of the renal artery and vein to the internal iliac artery and external iliac vein, respectively. For enteric drainage, the duodenum of graft was anastomosed to the recipients' ileum approximately 50-cm proximal to the ileocecal junction by hand-sewn two-layer methods. For bladder drainage, duodenal anastomosis was done to the dome of the recipients' bladder, also by hand-sewn two-layer methods.
All of the patients received basiliximab as an induction therapy. Maintenance immunosuppression consisted of steroids, calcineurin inhibitors, and mycophenolate mofetil. Tacrolimus was started at 0.075 mg/kg twice a day and adjusted to a serum trough level of 8-12 ng/mL. Mycophenolate mofetil was given at an oral dose of 500 mg twice a day, and the doses were adjusted for hematologic conditions or adverse effects. Methylprednisolone was administrated intravenously during surgery and then tapered to 5 or 10 mg of oral prednisolone daily. All recipients received infection prophylaxis, including intravenous 2nd generation cephalosporin continued for 3 days and then sulfamethoxazole/trimethoprim for 6 months. An oral suspension of antifungal agent was continued for 3 months postoperatively. Anticoagulation therapy was not routinely performed.
Delayed graft function of the kidney was defined as the need for dialysis in the first week posttransplant. Delayed graft function of the pancreas was defined by the patients' need for exogenous insulin at the time of hospital discharge [8].
After discharge, the patients were regularly followed up at the outpatient clinic. When an unexplained and sustained increase in serum creatinine or hyperglycemia was detected, evaluation of the kidney and the pancreas by duplex sonography and a biopsy of the kidney were recommended. Biopsy-proven T-cell mediated rejections were treated using steroid pulse therapy with 500 mg of methylprednisolone for 3 days.
Continuous data were summarized as the median with the range, and categorical data were summarized as proportions and percentages. The Kaplan-Meier method was used to calculate graft and patient survival rates. All statistical analyses were performed with the PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA).
Eight patients underwent SPK transplantation in our institute between January 2002 and June 2013. The median age of the patients was 35 years (range, 28-42 years). Three male and 5 female patients were included. All, except for one patient, were diagnosed with type I diabetes mellitus. A 35-year-old male patient became insulin-dependent after undergoing a total pancreatectomy because of traumatic injury 18 years ago. The median duration after diagnosis with diabetes mellitus was 19 years (range, 14-23 years). At the time of the operation, 4 patients had hypertension, 4 patients had retinopathy, and 3 patients had gastropathy as complications of the diabetes. No patients had a history of coronary artery disease or extremity amputation. The median duration of dialysis was 58 months (range, 41-72 months). Six patients were maintained on hemodialysis and the other 2 patients were on peritoneal dialysis.
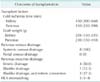
The median age of the donors was 38 years, from the youngest donor of 10 years old to the oldest donor of 49 years old. There were 6 male and 2 female donors. The causes of death were cranial trauma in 3, hypoxic brain damage in 2, and cerebrovascular accident in 3 donors. The median body mass index of the donors was 24.5 kg/m2 (range, 20.0-25.0 kg/m2). The median level of serum creatinine and amylase at procurement was 1.0 mg/dL (range, 0.5-1.2 mg/dL) and 94 IU/L (range, 22-260 IU/L), respectively. The median procurement pancreas suitability score was 16 points (range, 13-17 points). This score was defined by the Eurotransplant Pancreas Advisory Committee based on nine objective clinical parameters available at time of donor reporting [7,9]. The baseline characteristics of the donors and recipients are summarized in Table 1.
Implantations of the pancreas were all performed by the method of systemic venous drainage. Pancreas exocrine drainage was performed by enteric drainage in 4 patients and bladder drainage in 4 patients. In 3 of the 4 patients, the initial bladder drainage was converted to enteric drainage at 53, 54, and 208 days after transplantation, respectively. The reasons for enteric conversion were urinary fistula, hematuria, and reflux pancreatitis, respectively. The median cold ischemia time was 450 minutes (range, 300-600 minutes) for the kidney and 510 minutes (range, 330-590 minutes) for the pancreas. The median graft weight was 204 g (range, 150-235 g) for the kidney and 230 g (range, 150-410 g) for the pancreas. The operation-related characteristics are summarized in Table 2.
The demographic, surgical methods and outcomes of each patient are summarized in Table 3. Four patients required at least one relaparotomy. The first patient (patient No. 1) experienced 3 additional surgical procedures. The first relaparotomy was performed because of hemorrhage on the day after the transplantation. The second one was performed due to ureteral obstruction caused by ischemic change. Ureteral stent insertion was tried first, but failed, and a percutaneous nephrostomy and urteroureterostomy were performed 22 days after the initial operation. He had suffered from recurrent reflux pancreatitis and required enteric conversion of pancreas exocrine drainage 204 days after the transplant. The second patient (patient No. 3) developed fever 3 weeks after the initial operation, and a CT scan showed a large abscess formation around the transplanted pancreas. She underwent a relaparotomy, and portal venous thrombosis in transplanted pancreas and extended abscess were found. She underwent a graft pancreatectomy 22 days after the initial operation. The third patient (patient No. 4) had persistent hematuria and needed enteric conversion of pancreas exocrine drainage 55 days after the initial operation. The fourth patient (patient No. 6) underwent 2 additional surgical procedures because of recurrent leakage of the duodenal segment. At the first relaparotomy, she underwent detachment of duodenal anastomosis which was originally drained to the bladder and duodenojejunostomy was performed. However, 2 days after the relaparotomy, bile leakage was suspected and she underwent repair of fistula and omentopexy of the duodenojejunostomy.
Delayed graft function of the kidney and pancreas occurred in one patient (12.5%). Biopsy proven acute T-cell mediated rejection occurred in one patient (12.5%), and the time from transplantation to diagnosis of rejection was 30 months. With a median follow-up of 76 months (range, 2-147 months), the death-censored graft survival rate for the kidney was 100% at 1, 3, 5, and 10 years. The death-censored graft survival rates for the pancreas were 85.7% at 1, 3, and 5 years and 42.9% at 10 years (Fig. 1). One patient experienced pancreas graft loss because of venous thrombosis, and the other patient restarted exogenous insulin therapy 114 months after transplantation due to newly diagnosed type II diabetes mellitus. The median serum creatinine levels were 1.1 mg/dL (range, 0.5-1.4 mg/dL), 1.0 mg/dL (range, 0.7-1.6 mg/dL), and 1.4 mg/dL (range 0.6-1.5 mg/dL) at 1, 3, and 5 years, respectively. The median hemoglobin A1c was 5.4% (range, 5.0%-5.4%), 5.4% (range, 5.0%-5.4%), and 5.5% (range, 5.1%-5.7%) at 1, 3, and 5 years, respectively. The patient survival rates were 87.5% at 1, 3, 5, and 10 years (Fig. 2). A 34-year-old female patient died of pneumonia 59 days after the transplantation. The outcomes of the transplantation are summarized in Table 4.
The current study analyzed the 10-year experience of SPK transplantation. Even though the number of patients was small, we showed the long-term results of SPK transplantation in our early period. The graft survival for the kidney was excellent and the graft survival for the pancreas was also acceptable. One patient died with functioning grafts and there were two graft losses of the pancreas at 22 days and 114 months after the transplants, respectively. Five patients successfully achieved insulin independence. Patient survival was also similar to the results from other centers. The reported outcomes of SPK for type I diabetes mellitus are 88%, 77%, and 69% for 5-year patient, kidney, and pancreas graft survival, respectively [10,11].
SPK transplantation has been accepted as the standard treatment for patients with insulin-dependent diabetes mellitus and end-stage renal disease, who can endure a major operation [12]. The survival benefit has been shown by many publications [13,14]. SPK transplants recipients could expect to live 15 years longer than patients on the waiting list and 10 years longer than their counterparts who received a deceased donor kidney-only transplant [15]. In addition to the survival benefit, SPK improves quality of life, prevents diabetic nephropathy recurrence, improves or stabilizes diabetic neuropathy and retinopathy [16,17,18].
The long-tem benefits of SPK transplantation should be weighed against the potential early morbidities. Even though there have been many improvements in surgical techniques and postoperative management during the last decade, many potential nonimmunologic complications associated with surgical procedures exist [6]. In addition, the surgical techniques of organ procurement and implantation affect the early results and graft loss greatly. The early postoperative period is still considered as a high risk period for a relaparotomy [3]. Gruessner et al. [19] reported that the mortality hazard during the first week after transplants was increased compared to patients who remained on the waiting lists.
Because of these reasons, SPK transplants have been performed in only a few transplantation centers in Korea, especially centered in large-volume hospitals. We performed our first SPK transplantation in January 2002 and maintained a transplant program including SPK transplants for more than 10 years.
Graft pancreatectomy was performed in a 29-year-old female patient because of graft venous thrombosis. Pancreas venous thrombosis is one of the hazardous complications which can result in graft loss. The reported incidence of venous thrombosis is 2%-14% [12,20,21]. Technical errors, hypercoagulable stage and low-flow circulation can be the cause of portal vein thrombosis of a pancreas graft, and a prolonged cold ischemia time, the cause of the donor death and the body max index of the donor and recipient are suggested as the risk factors for graft thrombosis [21]. Many centers use anticoagulation therapy immediately after the operation. As one of the efforts for early diagnosis of venous thrombosis, CT angiography has been routinely performed in some centers [22]. Even though the risk of bleeding is increased, early start of anticoagulation therapy is mandatory and active surveillance with imaging modality is also required. Duplex scan is safe without the use of contrast media, however, it has limitations because of the depth of the organ, the gas-filled bowel and heterogenous positioning of the pancreas in each case. CT angiography rather than duplex scan could be a choice for surveillance, especially in small-volume centers which have a lack of experienced radiologists.
Duodenal leakage is one of the potential complications after pancreas transplantation. Graft pancreatectomy should be considered to salvage life in the situation of duodenal segment leaks with abdominal sepsis or prolonged morbidity with non-healing leakage [23]. One of our patients had repeated duodenal leakage and underwent a relaparotomy twice and finally died from pneumonia. The ischemia of the duodenal segment caused by the injury of the inferior pancreaticoduodenal arcade during procurement was thought to be a possible reason for the repeated leakage. The procurement of the pancreas should be done as meticulously as possible with gentle handling and minimal touching.
There were 3 cases that required conversion from the initial bladder drainage to enteric drainage. In the past, bladder drainage was preferred to enteric drainage with the advantage of the ability to monitor pancreatic function. However, improvements in immunosuppressive medication, operative skills and postoperative care have made enteric drainage a primary choice in the procedure in most of the established transplant centers [24,25]. There are also favorable results for the conversion of bladder to enteric drainage without increased risk of graft loss [26]. However, when enteric conversion was performed in an emergent situation due to anastomosis leakage, the results could be worse than in the elective cases [1]. In our series, only the patient who underwent enteric conversion with duodenal leakage had recurrent duodenal leaks and peritonitis. Two other patients underwent enteric conversion without surgical complications with a 6-week and 6-month interval, respectively. If patients suffer from the genitourinary symptoms, it is not necessary to wait more than 6 months or 1 year until the risk of rejection is reduced.
One of the patients presented long standing gastroparesis even with good graft pancreatic function. He had been diagnosed with insulin-dependent diabetes for 15 years and diabetic gastropathy for 3 years. After the transplantation, he had recovered uneventfully and maintained normoglycemia, however, he was repeatedly admitted with symptoms of vomiting and abdominal pain, which were refractory to the prokinetic medications. There was no evidence of bowel obstruction in the esophagogastroscopy and CT scan. Finally, a scintigraphy with Tc-99m diethylene triamine pentacaetic acid labelled solid food was performed 3 years after the transplant and showed delayed gastric emptying. He became free from gastrointestinal symptoms 7 years after the transplant without any further rescue treatments. Gastrointestinal autonomic neuropathy occurs frequently in patients with diabetes and has a profound negative impact on quality of life. The symptoms could last a long time even after the transplant. It may be unrecognized, especially after transplantation, thus, a high index of suspicion of diabetic gastropathy is required.
The technical complication rate after pancreas transplants is higher than after most other solid organ transplants [21]. In our series, 4 out of 8 patients underwent a relaparotomy due to surgical complications. As a small-volume center with initial experience, we had continued troubleshooting the technical problems in the early period of pancreas transplantation. Other than technical factors, postoperative management with a multidisciplinary approach is also mandatory for successful pancreas transplantation. We had made an effort to accomplish a collaborative team-based approach with physicians, radiologist and pathologists. The outcomes of the current series were comparable to other large-scaled data, and we thought it is possible to start and establish a pancreas transplant program even in small-volume centers in Korea. With more experiences, the outcomes are expected to improve, and the solitary pancreas transplantation would be possible to perform.
In conclusion, many surgical problems in SPK transplants have been overcome and the long-term graft and patient survival in our current series are comparable to previous studies. Although there are many potential morbidities and technical difficulties in SPK transplants, a successful pancreas transplant program can be established in a small-volume institute. A meticulous technique of procurement and the early start of an anticoagulation therapy are mandatory to further improve the outcomes.
Figures and Tables
References
1. Kleespies A, Mikhailov M, Khalil PN, Preissler G, Rentsch M, Arbogast H, et al. Enteric conversion after pancreatic transplantation: resolution of symptoms and long-term results. Clin Transplant. 2011; 25:549–560.
2. Weiss AS, Smits G, Wiseman AC. Twelve-month pancreas graft function significantly influences survival following simultaneous pancreas-kidney transplantation. Clin J Am Soc Nephrol. 2009; 4:988–995.
3. Page M, Rimmele T, Ber CE, Christin F, Badet L, Morelon E, et al. Early relaparotomy after simultaneous pancreaskidney transplantation. Transplantation. 2012; 94:159–164.
4. Gross CR, Limwattananon C, Matthees B, Zehrer JL, Savik K. Impact of transplantation on quality of life in patients with diabetes and renal dysfunction. Transplantation. 2000; 70:1736–1746.
5. Reddy KS, Stablein D, Taranto S, Stratta RJ, Johnston TD, Waid TH, et al. Long-term survival following simultaneous kidney-pancreas transplantation versus kidney transplantation alone in patients with type 1 diabetes mellitus and renal failure. Am J Kidney Dis. 2003; 41:464–470.
6. Ojo AO, Meier-Kriesche HU, Hanson JA, Leichtman A, Magee JC, Cibrik D, et al. The impact of simultaneous pancreas-kidney transplantation on long-term patient survival. Transplantation. 2001; 71:82–90.
7. Woeste G, Moench C, Hauser IA, Geiger H, Scheuermann E, Bechstein WO. Can the preprocurement pancreas suitability score predict ischemia-reperfusion injury and graft survival after pancreas transplantation? Transplant Proc. 2010; 42:4202–4205.
8. Tan M, Kandaswamy R, Sutherland DE, Gruessner RW, Gruessner AC, Humar A. Risk factors and impact of delayed graft function after pancreas transplants. Am J Transplant. 2004; 4:758–762.
9. Vinkers MT, Rahmel AO, Slot MC, Smits JM, Schareck WD. How to recognize a suitable pancreas donor: a Eurotransplant study of preprocurement factors. Transplant Proc. 2008; 40:1275–1278.
10. Young BY, Gill J, Huang E, Takemoto SK, Anastasi B, Shah T, et al. Living donor kidney versus simultaneous pancreas-kidney transplant in type I diabetics: an analysis of the OPTN/UNOS database. Clin J Am Soc Nephrol. 2009; 4:845–852.
11. Sampaio MS, Kuo HT, Bunnapradist S. Outcomes of simultaneous pancreas-kidney transplantation in type 2 diabetic recipients. Clin J Am Soc Nephrol. 2011; 6:1198–1206.
12. Banga N, Hadjianastassiou VG, Mamode N, Calder F, Olsburgh J, Drage M, et al. Outcome of surgical complications following simultaneous pancreas-kidney transplantation. Nephrol Dial Transplant. 2012; 27:1658–1663.
13. Sollinger HW, Odorico JS, Becker YT, D'Alessandro AM, Pirsch JD. One thousand simultaneous pancreas-kidney transplants at a single center with 22-year follow-up. Ann Surg. 2009; 250:618–630.
14. Pruijm MT, de Fijter HJ, Doxiadis II, Vandenbroucke JP. Preemptive versus Non-preemptive simultaneous pancreas-kidney transplantation: a single-center, long-term, follow-up study. Transplantation. 2006; 81:1119–1124.
15. Viglietti D, Serrato T, Abboud I, Antoine C, Pillebout E, Busson M, et al. Kidney graft dysfunction in simultaneous pancreas-kidney recipients after pancreas failure: analysis of early and late protocol biopsies. Clin Transplant. 2013; 27:E249–E255.
16. Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med. 1998; 339:69–75.
17. Allen RD, Al-Harbi IS, Morris JG, Clouston PD, O'Connell PJ, Chapman JR, et al. Diabetic neuropathy after pancreas transplantation: determinants of recovery. Transplantation. 1997; 63:830–838.
18. Wilczek HE, Jaremko G, Tyden G, Groth CG. Evolution of diabetic nephropathy in kidney grafts. Evidence that a simultaneously transplanted pancreas exerts a protective effect. Transplantation. 1995; 59:51–57.
19. Gruessner RW, Sutherland DE, Gruessner AC. Mortality assessment for pancreas transplants. Am J Transplant. 2004; 4:2018–2026.
20. Steurer W, Malaise J, Mark W, Koenigsrainer A, Margreiter R. Euro-SPK Study Group. Spectrum of surgical complications after simultaneous pancreas-kidney transplantation in a prospectively randomized study of two immunosuppressive protocols. Nephrol Dial Transplant. 2005; 20:Suppl 2. ii54–ii62.
21. Humar A, Ramcharan T, Kandaswamy R, Gruessner RW, Gruessner AC, Sutherland DE. Technical failures after pancreas transplants: why grafts fail and the risk factors: a multivariate analysis. Transplantation. 2004; 78:1188–1192.
22. Kim YH, Park JB, Lee SS, Byun JH, Kim SC, Han DJ. How to avoid graft thrombosis requiring graftectomy: immediate post-transplant CT angiography in pancreas transplantation. Transplantation. 2012; 94:925–930.
23. Singh RP, Vrakas G, Hayek S, Hayek S, Anam S, Aqueel M, et al. Clinically significant peripancreatic fluid collections after simultaneous pancreas-kidney transplantation. Transplantation. 2013; 95:1263–1269.
24. Monroy-Cuadros M, Salazar A, Yilmaz S, McLaughlin K. Bladder vs enteric drainage in simultaneous pancreas-kidney transplantation. Nephrol Dial Transplant. 2006; 21:483–487.
25. Sutherland DE, Gruessner RW, Dunn DL, Matas AJ, Humar A, Kandaswamy R, et al. Lessons learned from more than 1,000 pancreas transplants at a single institution. Ann Surg. 2001; 233:463–501.
26. Jimenez-Romero C, Manrique A, Morales JM, Lopez RM, Morales E, Cambra F, et al. Conversion from bladder to enteric drainage for complications after pancreas ransplantation. Transplant Proc. 2009; 41:2469–2471.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download