Abstract
Purpose
To determine the long-term outcomes of patients with diabetes mellitus (DM) and tissue loss who have undergone infrainguinal bypass surgery (IBS).
Methods
We retrospectively reviewed the medical records of 91 patients with DM and tissue loss who underwent IBS between July 2003 and December 2013. We determined the rates of overall survival (OS), amputation-free survival (AFS), limb salvage (LS), and graft patency (GP). In addition, we evaluated data to identify risk factors that affected long-term outcomes.
Results
The mean age of patients was 66 ± 8 years, and 78 patients (85.7%) were men. The locations of tissue loss were toe on 76 limbs (71.6%), heel on 6 limbs (5.7%) and others on 24 limbs (22.6%). Single lesions were found in 81 limbs (76.4%). According to categorization by distal anastomosis artery, there were 57 popliteal (53.8%) and 49 infrapopliteal bypasses (46.2%). Among infrapopliteal bypasses, 5 cases (10.2%) were sequential bypasses. The OS at 1, 3, and 5 years was 90.5%, 70.9%, and 44.2%, respectively. At 1, 3, and 5 years, the LS was 92.1%, 88.9%, 88.9%, respectively; and AFS was 84.4%, 67.6%, 45.7%, respectively. At 1, 3, and 5 years, the GP was 84.8%, 74.5%, and 69.8%, respectively. Renal failure was a negative predictor for OS, and female gender was a negative predictor for GP.
The prevalence of foot ulceration has been estimated to be 15% in patients with diabetes mellitus (DM) [1]. Diabetics with foot ulcers and chronic limb ischemia (CLI) have higher rates of major amputation and mortality than nondiabetics [2,3]. Therefore, the diabetic patient should receive multidisciplinary care, including active revascularization, to reduce the risk of major amputation. Most patients with DM and CLI have occlusive lesions in their tibial arteries and relatively intact pedal arteries. Bypass surgery using a vein graft to the crural or pedal arteries was regarded as the gold standard of revascularization for patients with DM and CLI [4]. However, because of new techniques and devices, the number of endovascular revascularization procedures has increased, and endovascular revascularization is performed frequently for high-risk patients, including those with DM and comorbidities such as renal insufficiency (RI) or cardiovascular disease. Percutaneous transluminal angioplasty (PTA) has had acceptable rates of limb salvage (LS), but the rates of technical success and primary patency have not been as high as those of bypass surgery [5,6]. The aim of this study was to determine the long-term outcomes and factors that led to poor results for patients with DM and tissue loss who underwent infrainguinal bypass surgery (IBS).
From July 2003 to December 2013, there were 269 patients with peripheral artery occlusive disease who underwent IBS at Kyungpook National University Hospital. Among the 116 patients with DM, 91 patients (106 limbs) were included in this retrospective study. Patients with other causes of occlusive disease, such as thromboembolism and vasculitis, were excluded. The following variables from the medical records were evaluated: age at the time of operation, gender, comorbidities, lesion characteristics, and the type of procedure (anastomotic distal target artery and conduit).
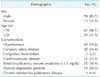
The duration of mean follow-up was 36.2 ± 30.1 months. The mean age of the patients was 66.3 ± 7.8 years (median, 66 years; range, 47 to 83 years), and there were 78 male patients (85.7%) (n = 91). Hypertension was present in 49 patients (59.8%) and coronary artery disease in 20 (24.4%). There were 25 patients (30.4%) with RI (serum creatinine ≥ 1.5 mg/dL). Among these patients, 19 (20.9%) were dependent on dialysis (Table 1).
Tissue loss consisted of ulcerative lesions in 80 limbs (75.5%) and gangrenous lesions in 26 limbs (24.5%). The most common site of wound was the toe (76 limbs, 71.7%). Heel site wound was in 6 limbs (5.7%) and other site wound was in 24 limbs (22.6%). Single lesions were found in 81 limbs (76.4%) and multiple lesions in 25 limbs (23.6%).
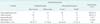
According to the categorization by distal anastomosis artery, there were 57 popliteal (53.8%) and 49 infrapopliteal bypasses (46.2%). Among the infrapopliteal bypasses, 5 cases (10.2%) were sequential bypasses. The most common site of inflow artery was the common femoral artery (82 limbs, 77.4%). The other sites were external iliac artery in 8 popliteal arteries (7.5%) and in 14 limbs (13.2%). Among the two remaining cases, one case was superficial femoral artery and the other case was tibioperoneal trunk artery. Among the patients undergoing infrapopliteal bypass, 13 (12.3%) received a pedal bypass, and the anastomotic distal artery was considered to be the paramalleolar posterior tibial artery, plantar artery, or dorsalis pedis artery.
The great saphenous vein (GSV) was used as graft in 58 limbs (54.7%), and among these, there were 42 cases of infrapopliteal bypass. Among 58 bypasses using GSV, a reverse translocation method was performed in 47 limbs (81.0%) and in situ was in 11 limbs (19.0%). A prosthetic graft was used for 40 limbs (37.7%). All prosthetic grafts were used for popliteal bypass, and most were for above-the-knee (AK) popliteal bypass (37 limbs). One infrapopliteal bypass procedure was performed using a graft that consisted of GSV spliced with an arm vein, and one infrapopliteal bypass procedure was performed using an arm vein as the graft. All sequential bypasses consisted of AK popliteal bypass using a prosthetic graft and then a short GSV graft for below-the-knee (BK) bypass (Table 2).
The endpoints were rates of overall survival (OS), amputation-free survival (AFS), LS, and graft patency (GP). OS was defined as the time from IBS to death from any cause. AFS was defined as the time from IBS to death or major amputation (above or below knee), and LS was defined as the time from IBS to major amputation. GP was defined as the time from IBS (considered to be primary patency) to any intervention to maintain or reestablish patency. We evaluated patency of graft by duplex ultrasound color-flow scan or Doppler plethysmography with measurement of ankle-brachial pressure index according to reporting standards [7].
The endpoints were estimated using Kaplan-Meier analysis and log-rank test. The Cox proportional hazards model was used to evaluate variables. A P-value less than 0.05 was considered statistically significant.
The OS at 1, 3, and 5 years was 90.5%, 70.9%, and 44.2%, respectively. There was no postoperative mortality. RI was found to be a negative factor for OS. The OS at 1, 3, and 5 years in patients without RI was 93.4%, 80.8%, and 52.2%, respectively; whereas for patients with RI, the OS was 75.4%, 48.8%, and 27.1%, respectively (P = 0.009). Among the 29 patients (55.2%) with RI, 16 died during follow-up. The mean duration from IBS to the death of these patients was 21.3 ± 15.9 months.
The AFS for all patients at 1, 3, and 5 years was 84.4%, 67.6%, and 45.7%, respectively. The AFS of patients with RI was 69.3%, 43.4%, and 31.0% at 1, 3, and 5 years, respectively; which was lower than the AFS for patients without RI (P = 0.014) (90.7%, 78.3%, and 52.6% at 1, 3, and 5 years).
The LS for all patients at 1, 3, and 5 years was 92.1%, 88.9%, and 88.9%, respectively. Gender was found to affect LS. Women had lower LS than men. The LS for women at 1, 3, and 5 years was 72.9%, 72.9%, and 72.9%, respectively (P = 0.005). Dialysis-dependent patients had lower LS than nondialysis patients. The LS for dialysis-dependent patients at 1, 3, and 5 years was 76.7%, 76.7%, and 76.7%, respectively (P = 0.031).
Other variables such as types of bypass and conduits and lesion characteristics did not affect LS. Major amputations were performed on 10 limbs (9.4%). Eight limbs underwent BK amputation and 2 limbs underwent AK amputation. These were performed in 6 limbs of 76 limbs (7.9%) with toe wound, 1 of 6 limbs (16.7%) with heel wound and 3 of 24 limbs (12.5%) with other wound locations. Nine of the 10 limbs (90.0%) underwent major amputation because of an extensive infected lesion or uncontrolled infection and the remaining 1 limb (10.0%) underwent AK amputation because of uncontrolled osteomyelitis of the tibia and overlying skin necrosis.
The GP for all patients at 1, 3, and 5 years was 84.8%, 74.5%, and 69.8%, respectively. Female gender negatively affected GP. The GP of male patients at 1, 3, and 5 years was 89.5%, 79.0%, and 72.9%, respectively; and for female patients was 60.0%, 50.0%, and 50.0%, respectively (P = 0.001). There was no difference between the GP of popliteal and the GP of infrapopliteal bypass procedures (Fig. 1). The GP at 1, 3, and 5 years for bypasses using a single GSV graft was 84.8%, 78.2%, and 78.2%, respectively. The GP of prosthetic graft was 88.9%, 77.9% and 69.2% at 1, 3, and 5 years. The GP of sequential bypass was 60.0% at 1, 3, and 5 years.
By multivariate analysis, RI was a negative risk factor for OS (P = 0.011), and female gender was a negative factor for GP (P = 0.022) (Fig. 2). Other variables were not significant influencing factors.
Revascularization in patients with DM can be difficult because of multilevel occlusive arterial lesions and calcified blood vessels. The OS and LS of patients with DM and peripheral arterial disease (PAD) have been reported to be 50% at 5 years and 85% at 1 year, respectively [8,9]. In addition, patients with coexisting chronic kidney disease have had poor long-term survival following revascularization [10], which is similar to the findings of our study.
There are no available data from randomized trials comparing bypass surgery and endovascular intervention for DM patients. PTA with successful revascularization has equivalent LS to bypass surgery [11,12]. However, the technical success and subsequent durability of PTA are limited compared with bypass surgery. Someone presented the primary patency of infrapopliteal PTA at 1 and 5 years was 57% and 38% [13]. Another author presented the primary, assisted primary and secondary patency of PTA at 3 years was 23.5%, 41.8%, and 46.1%, respectively [14]. Although PTAs can be repeated, they are not without risk [10]. PTA is going to replace a large part of the bypass, but still has limitation to patency. Evidence determining which procedure should be attempted first, PTA or bypass, is limited. The available data suggest that the "bypassfirst" strategy was associated with a trend toward improved OS and AFS in patients who survived at least two years [15]. Conte [4] has proposed that the decision on type of procedure should integrate several factors, including the patient's surgical risk, life expectancy, severity of ischemia, arterial anatomy, and conduit availability.
The annual major amputation rate of patients with diabetic foot ulcer (DFU) is 5% to 8%, and 85% of major amputations are preceded by a severe infection or gangrene [16,17,18]. In our study, major amputations were performed on 10 limbs (9.4%). Nine of these limbs (90.0%) had extensive infectious lesions or uncontrolled infections. Although successful revascularization had been performed, and the grafts remained patent; major amputation was performed because of severe soft tissue infection. Therefore, not only revascularization but also infection control measures, including drainage and minor amputation, are important procedures for the diabetic foot [19].
The common etiologies of DFU are neuropathy, trauma, deformity, high pressure on the plantar foot, and PAD [20,21]. DM vasculopathy is characterized by increased thickness of the arterial media due to increased deposition of extracellular matrix and increased collagen, as well as proliferation of endothelial and adventitial cells [22,23]. Diabetic PAD is characterized by involvement of medium-sized arteries such as the popliteal or tibial arteries, and preservation of the distal pedal vessels [24]. There are also functional abnormalities of the microcirculation [25]. Therefore, the features of PAD in patients with DM lead to impeded healing of DFUs, regardless of successful revascularization of the lower extremity [26].
Infrapopliteal bypass using prosthetic conduit had significantly higher 30-day failure rates [27]. In our study, there was no significant difference in GP between single GSV and prosthetic graft. For that reason, we thought it was because all artificial grafts were used in popliteal bypass, especially in AK popliteal bypass (37 of 40 limbs), whereas a single GSV was used mainly in distal bypass.
A shortcoming of our study was that we did not verify that our patients had characteristic DM vasculopathy. Thus, we could not confirm if the lesions of our patients were DFUs, and cannot state with certainty that our results represent the outcomes of infrainguinal bypass for patients with DFU. Additional research that verifies that PAD is present in patients with DM and tissue loss is needed.
In conclusion, IBS for patients with DM and tissue loss led to acceptable OS, AFS, LS, and GP. Women had lower GP after the procedure. Because variations in the procedural aspects of IBS did not negatively affect outcomes, we believe that it is not necessary to avoid performing long bypasses such as crural or pedal bypasses, if the target vessels are adequate and conduits are available. Active revascularization for patients with DM and tissue loss can reduce the risk of major amputation. However, for patients with DM and RI, who in our study had reduced OS, an endovascular procedure should be considered instead of bypass surgery. In addition, effective infection control is also important as well as active revascularization for LS.
Figures and Tables
Fig. 1
(A) Limb salvage rate of groups classified by distal anastomotic artery was not significantly different (P = 0.059). a, above the knee popliteal bypass; b, below the knee popliteal bypass; c, crural bypass; d, pedal bypass. (B) Graft patency of groups classified by distal anastomotic artery was not significantly different (P = 0.637) a, above the knee popliteal bypass; b, below the knee popliteal bypass; c, crural bypass; d, pedal bypass.

References
1. Faries PL, Teodorescu VJ, Morrissey NJ, Hollier LH, Marin ML. The role of surgical revascularization in the management of diabetic foot wounds. Am J Surg. 2004; 187(5A):34S–37S.
2. Global Lower Extremity Amputation Study Group. The Global Lower Extremity Amputation Study Group. Epidemiology of lower extremity amputation in centres in Europe, North America and East Asia. Br J Surg. 2000; 87:328–337.
3. Carmona GA, Hoffmeyer P, Herrmann FR, Vaucher J, Tschopp O, Lacraz A, et al. Major lower limb amputations in the elderly observed over ten years: the role of diabetes and peripheral arterial disease. Diabetes Metab. 2005; 31:449–454.
4. Conte MS. Diabetic revascularization: endovascular versus open bypass: do we have the answer? Semin Vasc Surg. 2012; 25:108–114.
5. Cooper JC, Welsh CL. The role of percutaneous transluminal angioplasty in the treatment of critical ischaemia. Eur J Vasc Surg. 1991; 5:261–264.
6. Wahlgren CM, Kalin B, Lund K, Swedenborg J, Takolander R. Long-term outcome of infrainguinal percutaneous transluminal angioplasty. J Endovasc Ther. 2004; 11:287–293.
7. Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997; 26:517–538.
8. Hinchliffe RJ, Andros G, Apelqvist J, Bakker K, Friederichs S, Lammer J, et al. A systematic review of the effectiveness of revascularization of the ulcerated foot in patients with diabetes and peripheral arterial disease. Diabetes Metab Res Rev. 2012; 28:Suppl 1. 179–217.
9. Moulik PK, Mtonga R, Gill GV. Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care. 2003; 26:491–494.
10. Owens CD, Ho KJ, Kim S, Schanzer A, Lin J, Matros E, et al. Refinement of survival prediction in patients undergoing lower extremity bypass surgery: stratification by chronic kidney disease classification. J Vasc Surg. 2007; 45:944–952.
11. Romiti M, Albers M, Brochado-Neto FC, Durazzo AE, Pereira CA, De Luccia N. Meta-analysis of infrapopliteal angioplasty for chronic critical limb ischemia. J Vasc Surg. 2008; 47:975–981.
12. Albers M, Romiti M, Brochado-Neto FC, De Luccia N, Pereira CA. Meta-analysis of popliteal-to-distal vein bypass grafts for critical ischemia. J Vasc Surg. 2006; 43:498–503.
13. Lo RC, Darling J, Bensley RP, Giles KA, Dahlberg SE, Hamdan AD, et al. Outcomes following infrapopliteal angioplasty for critical limb ischemia. J Vasc Surg. 2013; 57:1455–1463.
14. Kudo T, Chandra FA, Ahn SS. The effectiveness of percutaneous transluminal angioplasty for the treatment of critical limb ischemia: a 10-year experience. J Vasc Surg. 2005; 41:423–435.
15. Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: an intention-to-treat analysis of amputation-free and overall survival in patients randomized to a bypass surgery-first or a balloon angioplasty-first revascularization strategy. J Vasc Surg. 2010; 51:5 Suppl. 5S–17S.
16. Apelqvist J, Larsson J, Agardh CD. Long-term prognosis for diabetic patients with foot ulcers. J Intern Med. 1993; 233:485–491.
17. Prompers L, Schaper N, Apelqvist J, Edmonds M, Jude E, Mauricio D, et al. The EURODIALE Study. Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. Diabetologia. 2008; 51:747–755.
18. Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005; 366:1719–1724.
19. Kota SK, Kota SK, Meher LK, Sahoo S, Mohapatra S, Modi KD. Surgical revascularization techniques for diabetic foot. J Cardiovasc Dis Res. 2013; 4:79–83.
20. Reiber GE, Vileikyte L, Boyko EJ, del Aguila M, Smith DG, Lavery LA, et al. Causal pathways for incident lower-extremity ulcers in patients with diabetes from two settings. Diabetes Care. 1999; 22:157–162.
21. Frykberg RG. Diabetic foot ulcers: pathogenesis and management. Am Fam Physician. 2002; 66:1655–1662.
22. Rumble JR, Cooper ME, Soulis T, Cox A, Wu L, Youssef S, et al. Vascular hypertrophy in experimental diabetes. Role of advanced glycation end products. J Clin Invest. 1997; 99:1016–1027.
23. Vranes D, Cooper ME, Dilley RJ. Cellular mechanisms of diabetic vascular hypertrophy. Microvasc Res. 1999; 57:8–18.
24. LoGerfo FW, Coffman JD. Current concepts. Vascular and microvascular disease of the foot in diabetes. Implications for foot care. N Engl J Med. 1984; 311:1615–1619.
25. Sandeman DD, Pym CA, Green EM, Seamark C, Shore AC, Tooke JE. Microvascular vasodilatation in feet of newly diagnosed non-insulin dependent diabetic patients. BMJ. 1991; 302:1122–1123.
26. Arora S, Pomposelli F, LoGerfo FW, Veves A. Cutaneous microcirculation in the neuropathic diabetic foot improves significantly but not completely after successful lower extremity revascularization. J Vasc Surg. 2002; 35:501–505.
27. Nguyen BN, Neville RF, Abugideiri M, Amdur R, Sidawy AN. The effect of graft configuration on 30-day failure of infrapopliteal bypasses. J Vasc Surg. 2014; 59:1003–1008.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download