Abstract
In patients highly suspected of developing steal syndrome, the subscapular artery may be a good supplier for functional prosthetic arteriovenous access, as well as a good solution for the prevention of steal syndrome. A 51-year-old woman was preparing to have a loop shaped polytetrafluoroethylene (PTFE) graft placed at the left upper extremity. The diameter of subscapular the artery was 3 mm. Arterial calcification was not evident. The diameter of the basilic vein was 6 mm. A 50-cm long 4-7 mm tapered PTFE graft was placed in a loop shape between both skin incisions. The patient was uneventfully discharged at postoperative day 4 without any remaining steal syndrome. The PTFE graft was well-functioning during the follow-up period. The patient did not experience symptoms of steal syndrome any longer.
After arteriovenous vascular access creation for hemodialysis, steal phenomenon (a physiologic response to resistance differences) occurs in the majority of patients. Among these patients, serious complications that lead to surgical treatment appear in 3.7% to 5% of dialysis patients [1]. Risk factors for dialysis access related steal syndrome (DASS) include atherosclerosis, diabetes, old age, female gender and upper arm fistula versus lower arm, multiple operations in same limb, previous steal syndrome and use of prosthetic graft [2]. Symptoms range from mild numbness or discoloration to pain at rest and gangrene. In severe cases, closure of arteriovenous fistula (AVF) with ligation and loss of vascular access occurs. Procedures to treat DASS while maintaining a functional access include banding (flow restriction), revision using distal flow, distal revascularization and interval ligation [3] and proximalization of arterial inflow (PAI).
One of the important aspects is preventing the occurrence of DASS. The likelihood of DASS should be determined with each access procedure based on the clinical predictors. All preventative strategies to reduce the incidence should be implemented. All potential inflow arterial stenosis should be identified and corrected. Operative procedures should be selected or designed to minimize the risk of ischemia in patients deemed at risk. The risk of hand ischemia is much less for proximal radial artery based procedures compared to those originating from the brachial artery. For brachial artery based procedures, the incidence of hand ischemia likely decreases as the anastomosis is more cephalad. In a patient with a history of DASS that required a brachioaxillary prosthetic access, we constructed an upper arm PTFE graft using the high axillary branch artery. Most importantly, it is not a salvage procedure for DASS. This is a preliminary report about new access creation using subscapular artery as inflow for prevention of developing hand ischemia in a patient with a history of DASS.
A 51-year-old woman was diagnosed with end stage renal disease originated from long standing diabetes. The patient initially started hemodialysis with a tunneled cuffed hemodialysis catheter via the right internal jugular vein. For permanent hemodialysis vascular access, brachial-cephalic AVF was created at the patient's left upper extremity. However, the patient did not tolerate developing DASS. After the hand ischemia recovered by AVF ligation, the patient was referred to our hospital for difficult vascular access creation.
At the initial physical examinations, a mild tingling sensation of left hand remained without any hand ischemic symptoms, such as paleness, coldness, color change or gangrene. However, pulsation of radial and ulna artery was difficult to palpate at both upper arms. Allen test was positive at both hands. Selective arteriography of both upper arms was performed preoperatively. There were only interosseous arteries without radial or ulna arteries at bilateral distal upper extremeties. Additionaly, a brachial artery stenosis at the left elbow area was made owing to previous AVF creation. Following both ascending arm venograms revealed no abnormal finding at both upper extremity veins (Fig. 1). We wanted to create vascular access at the upper extremity considering her relatively young age. We decided to use the high axillary artery's branch as the inflow artery. In the right upper arm, hemodialysis catheter was placed at right anterior chest positioned near the right axilla area; it seemed to be difficult to approach at axilla area. Finally, the planned operation was to place a loop shaped polytetrafluoroethylene (PTFE) graft at the left upper extremity.
Under general anesthesia, a 7-cm skin incision was made at the axilla. The selected artery and connecting vein were isolated from the surrounding tissue. The diameter of the subscapular artery was 3 mm. Arterial calcification was not evident. The diameter of the basilic vein was 6 mm. For subcutaneous tunneling and graft anastomosis, an additional small skin incision was created above the elbow. A 50-cm long 4-7 mm tapered PTFE graft was placed in a loop shape between both skin incisions. A graft end-to-vein side anastomosis was performed first, with a graft end-to-arterial side anastomosis performed later (Fig. 2A, B). During the operation, pulse wave and O2 saturation were monitored by digital pulse oxymetry and maintained, perioperatively. Total operation time from skin to skin was 120 minutes. The patients received close suction drain at the axillary operative site, which was removed at 3 days postoperative. The patient was uneventfully discharged at postoperative day 4 without any remaining DASS. The PTFE graft was well functioning during the follow-up period. At postoperative month 8, percutaneous angioplasty was performed for stenosis at graft-venous anastomosis (Fig. 2C) site. The patient did not experience symptoms of steal syndrome any longer.
Prevention and minimization of the risk of steal syndrome is the best way to reduce serious complications. The most common way to prevent steal syndrome is using the radial artery to create a fistula, such as radiocephalic AVF. The incidence of steal syndrome in the radiocephalic AVF is 1%-2% [4]. However, if it is not possible to use the radial artery due to arterial disease or venous outflow problems, a more proximal artery is used that increases the incidence of steal syndrome to 6%-12%. The incidence rises in patients having upper extremity arterial disease [4]. In such cases, another approach to prevent ischemia is to move the arterial anastomosis from the brachial artery to the axillary artery to create an upper arm loop access [5]. The axillary artery is useful in patients at high risk of steal syndrome because of relatively large diameter and reduced involvement from atherosclerosis [6]. Based on these advantages, we decided to use the branch of the axillary artery, namely the subscapular artery. Satisfactory results were obtained. After creation of PTFE graft using subscapular artery in a patient with a history of DASS, the graft functioned well without any ischemic hand symptoms during the follow-up period.
The subscapular artery, the largest branch of the axillary artery, arises at the lower border of the subscapularis muscle. It branches to the latissimus dorsi, serratus anterior and subscapular muscles. The average calibre is approximately 4.5 mm, ranging from 3-7 mm. The distance from the origin of the subscapular artery to the major branch averages 8.2 cm [7]. A low incidence of atherosclerosis in the subscapular artery (8%) was reported in a study of 50 fresh cadaver dissections (mean age at death, 66 years) [8]. We also presently found an absence of atherosclerosis in the subscapular artery (diameter was 3 mm), which was sufficient for arterial anastomosis.
Using a branch of the high axilla artery, namely the subscapular artery, as inflow seems to have a similar effect with PAI, which can be used to treat DASS [9]. However, use of the subscapular artery as inflow has an additional appeal compared to PAI. It does not require using the axillary artery to anastomose. As a result, it does not disturb axial blood flow and prevents distal hand ischemia. In addition, through the axillary branch artery, larger and high-pressure artery supply of blood flow to distal extremities is possible. Mean systolic pressure in the axillary artery is significantly higher than that of the proximal brachial artery (153 mmHg vs. 116 mmHg, P = 0.007) [10].
However, there could be some drawbacks to this approach. Results of this procedure have not been reported anywhere before. It requires the use of a prosthetic PTFE graft, since the loop configuration requires a longer graft than other types of vascular access. This increases the risk of subsequent infection and thrombosis complications. The patient in this report experienced graft stenosis at 8 months postoperatively, which was treated by percutaneous angioplasty. The scapular artery lies deep in the axillary artery. Use of the artery requires sufficient skin incision for good field of vision and easy manipulation. In our case, the skin incision was 7 cm at the axilla area.
In conclusion, in patients under high risk of developing DASS, the subscapular artery may be a good supplier for functional prosthetic arteriovenous access and a good solution to prevent DASS.
Figures and Tables
Fig. 1
Preoperative arteriogram revealed arteriopathy of upper extremity. There were only interosseous arteries without radial or ulna arteries at bilateral distal upper extremities.
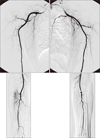
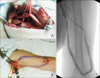
Fig. 2
(A) Subscapular artery was identified and dissected from adjacent structures with good field of vision at axillary approach (black arrow). (B) Loop shaped polytetrafluoroethylene graft was placed subcutaneously between two skin incisions. (C) At 8 months postoperatively, percutaneous angioplasty was done for stenosis of graft-venous anastomosis.
References
1. Tordoir JH, Dammers R, van der Sande FM. Upper extremity ischemia and hemodialysis vascular access. Eur J Vasc Endovasc Surg. 2004; 27:1–5.
2. Beathard GA, Spergel LM. Hand ischemia associated with dialysis vascular access: an individualized access flow-based approach to therapy. Semin Dial. 2013; 26:287–314.
3. Song D, Moon C. Surgical treatment, with using distal revascularization intervalligation, for the ischemia that follows creation of hemodialysis access. J Korean Surg Soc. 2008; 74:371–377.
4. Mickley V. Steal syndrome: strategies to preserve vascular access and extremity. Nephrol Dial Transplant. 2008; 23:19–24.
5. Gradman WS, Pozrikidis C. Analysis of options for mitigating hemodialysis access-related ischemic steal phenomena. Ann Vasc Surg. 2004; 18:59–65.
6. Jennings W, Brown R, Blebea J, Taubman K, Messiner R. Prevention of vascular access hand ischemia using the axillary artery as inflow. J Vasc Surg. 2013; 58:1305–1309.
7. Valnicek SM, Mosher M, Hopkins JK, Rockwell WB. The subscapular arterial tree as a source of microvascular arterial grafts. Plast Reconstr Surg. 2004; 113:2001–2005.
8. Bartlett SP, May JW Jr, Yaremchuk MJ. The latissimus dorsi muscle: a fresh cadaver study of the primary neurovascular pedicle. Plast Reconstr Surg. 1981; 67:631–636.
9. Jennings WC, Brown RE, Ruiz C. Primary arteriovenous fistula inflow proximalization for patients at high risk for dialysis access-associated ischemic steal syndrome. J Vasc Surg. 2011; 54:554–558.
10. Reifsnyder T, Arnaoutakis GJ. Arterial pressure gradient of upper extremity arteriovenous access steal syndrome: treatment implications. Vasc Endovascular Surg. 2010; 44:650–653.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download