Abstract
Purpose
Challenging iliac anatomy remains an important issue during endovascular aneurysm repair (EVAR), and it is known that the length of the common iliac artery (CIA) is shorter in Asians than in Western groups. We analyzed both the iliac anatomy and the incidence of adjunctive maneuvers to overcome iliac artery-related difficulties during EVAR.
Methods
Seventy-four EVARs were performed for abdominal aortic aneurysm between January 2010 and March 2013. Patient demographic data, iliac anatomical characteristics (presence of iliac artery aneurysm, iliac artery diameter and length, and iliac tortuosity), and adjunctive iliac artery maneuvers were reviewed retrospectively.
Results
Mean CIA length was 52.8 mm (range, 6.6-98.0 mm) on the right and 56.3 mm (range, 0-94.8 mm) on the left. CIA length was ≥20 mm, except in one patient with bilateral short CIAs. Forty patients (54%) had a CIA aneurysm, and 18 had aneurysms on both sides. Iliac adjunctive procedures were performed in 38 patients (51%) as follows: 23 internal iliac artery (IIA) embolizations or ligations, seven IIA revascularizations, 16 external iliac artery (EIA) balloon angioplasties or stenting, one EIA patch angioplasty, one EIA interposition, two femoral endarterectomies with patch angioplasty, and nine femoro-femoral bypasses after EVAR with an aorto-uni-iliac device. Technical success for the adjunctive iliac procedures was achieved in all patients.
The significance roles of the iliac artery during endovascular aortic aneurysm repair (EVAR) include an access site for a delivery system, a sealing zone for a stent graft, a source of pelvic flow and, occasionally, a combined iliac artery aneurysm that should be treated. Therefore, iliac anatomy is a major consideration during EVAR, and challenging iliac anatomy is remains a problem despite advancements in technology and devices [1,2]. Hostile iliac anatomy consists of a short common iliac artery (CIA), small ilio-femoral artery diameter, combined iliac artery aneurysm, and iliac tortuosity.
We analyzed iliac artery anatomy in patients with an abdominal aortic aneurysm (AAA) and investigated the types of iliac problems and adjunctive procedures associated with them during EVAR.
Eighty-one patients with AAA were treated in a tertiary hospital from January 2010 to March 2013. Seventy-four patients were included, and seven who underwent open surgical repair were excluded. The treatment of choice is EVAR. The reasons for open surgical repair were pararenal/juxtarenal AAA, Marfan syndrome, and hemorrhagic shock due to a ruptured AAA. Iliac anatomy was not a reason for open surgical repair. Our vascular registry and medical records were reviewed retrospectively.
All iliac anatomy was investigated by CT using Aquarius, iNtuition Ed ver 4.4.6 (TeraRecon Inc., Foster City, CA, USA). Vessel diameter was measured on images perpendicular to the central flow line (CFL). Diameter was defined as the length from the outer wall to the outer wall. However, luminal diameter (inner wall to inner wall) was measured for the external iliac artery (EIA), which was used to introduce a delivery system. An iliac artery aneurysm was defined as >20 mm. Artery length was measured on a stretched image along the CFL. The iliac tortuosity index was evaluated using a three-dimensional workstation (Fig. 1). Iliac tortuosity was graded from 0 to 3 using the tortuosity index (Table 1) [3].
All procedures were categorized into planned and unplanned procedures according to the reporting standard [4]. Indication of planned adjunctive procedures was that iliac anatomy was not suitable for instructions for use. Balloon angioplasty with or without stenting, patch angioplasty or interposition graft was used for iliofemoral occlusive disease. Internal iliac artery (IIA) embolization with stent graft extension to EIA was usually used to treat combined CIA aneurysm or short CIA. However, bilateral IIA embolization was not done to preserve pelvic flow and we tried to save at least one IIA. Regarding stent graft devices, Zenith (Cook Inc, Bloomington, IN, USA) was used in 63 patients and Excluder (W. L. Gore and Associates, Netwark, DE, USA) was used in 2 patients. When an aorto-uni-iliac (AUI) device was necessary in 9 patients, Endurant (Medtronic Inc, Santa Rosa, CA, USA) was used. Technical success for iliac adjunctive procedures was defined as successful introduction and deployment of a device without a type Ib or III endoleak or graft limb occlusion. Postoperative complications and mortality were investigated.
The patient demographic data are shown in Table 2. Mean patient age was 73 years, and 81% of the patients were male. Eleven patients had a ruptured AAA. Table 3 shows the details of the iliac anatomy. Mean CIA diameter was 20.8 mm on the right and 18.3 mm on the left. An aneurysm was found in 43% of right CIAs and 35% of left CIAs. Forty patients (54%) had at least one CIA aneurysm: 22 unilateral and 18 bilateral aneurysms. Mean CIA length was 52.8 mm on the right and 56.3 mm on the left. A short CIA (<20 mm) was found in one patient (Fig. 2). Thirty patients had unilateral or bilateral narrow EIA <7 mm. The iliac tortuosity grades are shown in Table 4. The mean iliac tortuosity grades were 1.4 on the right and 1.4 on the left. The most common grade was grade 1. Grade 3 was found in six (8%) on the right and two (3%) on the left.
Thirty-eight patients (51%) needed an adjunctive iliac procedure during the EVAR (Table 5). The most common procedure was IIA embolization to prevent an endoleak when an iliac limb was extended down to the EIA. Balloon angioplasty, stenting, patch angioplasty, and EIA interposition were performed in 18 patients for EIA stenosis; eleven patients, five patients, one patient, and one patient, respectively. In addition, endarterectomy with patch angioplasty for a stenotic common femoral artery was performed in two patients. Seven hybrid IIA revascularization procedures (six EIA to IIA bypasses and one IIA transposition to EIA) were done to preserve pelvic perfusion. An AUI device was used in nine patients who had narrow distal aortic diameter (<17 mm). The contralateral CIA was embolized with a vascular plug and a crossover femoro-femoral bypass was conducted.
One patient required a distal iliac limb extension to the EIA due to a type Ib endoleak. Four IIAs were unexpectedly covered without embolization; however, no IIA endoleaks occurred.
Technical success was achieved in 100% of patients who underwent an iliac adjunctive procedure, even though there were three type Ia endoleaks and 14 type II endoleaks. None needed open conversion. Other postoperative complications and mortality are described in Table 6.
Proper CIA length and diameter are necessary for a safe iliac landing zone. Within Asian anatomy, the short CIA is a difficult concern [5]. In a study based in Hong Kong, mean CIA lengths were 29.9 mm on the right and 34.2 mm on the left, compared to >50 mm in Caucasians. However, mean right and left CIA lengths in our study were 52.8 and 56.3 mm, respectively, which was not different from Western data. A short CIA (<20 mm) was detected in one patient (1%, 1/74) who had bilateral short CIAs. This problem was overcome using a hybrid procedure; EIA to IIA bypass with contralateral IIA embolization. Mean CIA diameters reported from another Korean study were 41.1 and 43.2 mm [6]. Thus, all Asians do not have short CIAs, and CIA length seemed suitable in most cases.
In the EUROSTAR (EUROpean collaborators on Stent-graft Techniques for abdominal aortic Aneurysm Repair) registry, mean CIA diameter was 16.7 mm, and the rate of concomitant uni or bilateral CIA aneurysms was only 16.8% (1,269/7,554) [7]. However, 54% of our patients had a uni- or bilateral CIA aneurysm. Mean CIA diameters were 20.8 mm on the right and 18.3 mm on the left. Previous Asian studies showed similar mean CIA diameters (17.9-20.9 mm) [5,6]. Therefore, a large CIA diameter rather than a short CIA are characteristics of Asian iliac anatomy.
Due to unsuitable CIA anatomy, including short length and large diameter, IIA embolization was the most common adjunctive procedure in this study. An iliac extension limb was deployed to the EIA after IIA embolization to prevent a type II endoleak. This is the easiest way to solve a CIA problem. Variable pelvic ischemic complications, including buttock claudication, erectile dysfunction, ischemic colitis, gluteal or perineal necrosis, and spinal cord ischemia have been reported after interrupting pelvic flow [8,9,10,11,12,13,14]. Thus, bilateral IIA embolization is seldom used to preserve pelvic perfusion.
We used a hybrid procedure, such as EIA to IIA bypass with contralateral IIA embolization, to overcome bilateral CIA problems. Although this additional bypass surgery enabled successful EVAR, retroperitoneal procedures are associated with greater blood loss, higher postoperative complication rates, and longer hospital stays [15]. Several methods have been introduced to treat those patients with an endovascular method, such as the bell-bottom technique, use of an iliac branch device (IBD), or a sandwich technique. The bell-bottom technique using a flared iliac stent graft is the simplest. We used this technique in 4 patients: two patients with ruptured AAA and two highrisk patients. The immediate result was good in all patients. However, there are concerns about type Ib endoleaks due to continued iliac artery enlargement [16,17]. In a recent study, no differences in reintervention rates for type Ib or III endoleaks were observed between the bell-bottom technique and IIA embolization with stent graft extension to the EIA during a median 22 month follow-up [18]. However, long-term data on the bell-bottom technique are needed.
IBD was designed to provide both sealing and blood flow into the EIA and IIA. The technical success rate of EVAR using IBD is 85%-100% and midterm patency is about 70% [19]. However, it is not available in all countries. Recently, domestic IBD was introduced in Korea, but its clinical data is absent yet. The sandwich technique is another option, which was first reported by Lobato [20]. Midterm follow-up results of 40 consecutive cases were announced recently; the technical success rate was 100%, and the primary patency rate was 93.8% during a mean followup of 12 months [21].
We did not experience tortuosity-related complications or any difficulties positioning stent grafts. Although superstiff guidewire with an introducer catheter usually allows a delivery system to pass a tortuous iliac artery, a stent graft delivery system may occasionally not pass, which is when a brachiofemoral wire becomes useful, as it improves trackability by placing tension on the wire and controlling both ends [22]. It is called the body floss technique. Iliac tortuosity may increase the risk of arterial dissection, rupture, limb kinking, or occlusion [23,24,25]. Therefore, the operator should pay attention to avoid complications when iliac tortuosity is severe, although iliac tortuosity is not an absolute contraindication for EVAR.
The last iliac problem is a small diameter iliac artery. Balloon angioplasty and stent placement are the simplest methods to overcome this problem. These methods can be performed simultaneously with EVAR. An iliac conduit is a good option when these methods are insufficient to achieve adequate iliac access [26]. An iliac conduit is used to introduce a delivery system and can be used as an ilio-femoral bypass conduit after EVAR. An aggressive endovascular solution called the "internal endoconduit" technique has been introduced to avoid retroperitoneal dissection [27]. After the endograft has been introduced into the iliac artery, a controlled iliac artery rupture is created, while maintaining proximal and distal sealing. However, this technique is not recommended routinely.
We used an AUI device in patients with a narrow distal aorta. An AUI device is useful when access to only one iliac artery is available. In a large series [28], an AUI device with femoro-femoral bypass grafting resulted in a lower technical success rate (94% vs. 99%, P = 0.002), a higher perioperative complication rate (10% vs. 4%, P = 0.005), and lower 3-year primary patency (92% vs. 98%, P = 0.003) compared with those of a bifurcated device. Three-year secondary patency in the AUI group was 97%. Although it was also lower than the bifurcated device group (97% vs. 99%, P = 0.040), it seemed acceptable. Therefore, EVAR with an AUI device is a good alternative treatment option for high-risk patients with hostile iliac anatomy.
Although challenging iliac artery anatomy is a major obstacle during EVAR, supplemental endovascular or hybrid procedures can facilitate endovascular treatment under those conditions. A precise assessment of iliac anatomy with adequate instruments and techniques is crucial. In our study, a short CIA was uncommon, and a large CIA diameter was more important during EVAR in Asians.
Figures and Tables
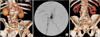
Fig. 1
Measuring the iliac tortuosity index. The iliac tortuosity index was defined by dividing L1 by L2, where L1 (A and B) was the distance along the central lumen line between the common femoral artery and the aortic bifurcation, and L2 (C) was the straight-line distance from the common femoral artery and the aortic bifurcation. In this case, the iliac tortuosity index was 1.65 (233/141).
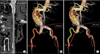
Fig. 2
A 70-year-old male presented with an abdominal aortic aneurysm (AAA) and bilateral short common iliac arteries (A and B). Both iliac stent graft limbs were deployed on external iliac arteries (EIAs). The left internal iliac artery (IIA) was embolized with coils, and the right IIA was perfused by the EIA to IIA bypass (C).
References
1. Murray D, Ghosh J, Khwaja N, Murphy MO, Baguneid MS, Walker MG. Access for endovascular aneurysm repair. J Endovasc Ther. 2006; 13:754–761.
2. Henretta JP, Karch LA, Hodgson KJ, Mattos MA, Ramsey DE, McLafferty R, et al. Special iliac artery considerations during aneurysm endografting. Am J Surg. 1999; 178:212–218.
3. Walker TG, Kalva SP, Yeddula K, Wicky S, Kundu S, Drescher P, et al. Clinical practice guidelines for endovascular abdominal aortic aneurysm repair: written by the Standards of Practice Committee for the Society of Interventional Radiology and endorsed by the Cardiovascular and Interventional Radiological Society of Europe and the Canadian Interventional Radiology Association. J Vasc Interv Radiol. 2010; 21:1632–1655.
4. Chaikof EL, Blankensteijn JD, Harris PL, White GH, Zarins CK, Bernhard VM, et al. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg. 2002; 35:1048–1060.
5. Cheng SW, Ting AC, Ho P, Poon JT. Aortic aneurysm morphology in Asians: features affecting stent-graft application and design. J Endovasc Ther. 2004; 11:605–612.
6. Park KH, Lim C, Lee JH, Yoo JS. Suitability of endovascular repair with current stent grafts for abdominal aortic aneurysm in Korean patients. J Korean Med Sci. 2011; 26:1047–1051.
7. Hobo R, Sybrandy JE, Harris PL, Buth J. EUROSTAR Collaborators. Endovascular repair of abdominal aortic aneurysms with concomitant common iliac artery aneurysm: outcome analysis of the EUROSTAR Experience. J Endovasc Ther. 2008; 15:12–22.
8. Criado FJ, Wilson EP, Velazquez OC, Carpenter JP, Barker C, Wellons E, et al. Safety of coil embolization of the internal iliac artery in endovascular grafting of abdominal aortic aneurysms. J Vasc Surg. 2000; 32:684–688.
9. Karch LA, Hodgson KJ, Mattos MA, Bohannon WT, Ramsey DE, McLafferty RB. Adverse consequences of internal iliac artery occlusion during endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 2000; 32:676–683.
10. Wolpert LM, Dittrich KP, Hallisey MJ, Allmendinger PP, Gallagher JJ, Heydt K, et al. Hypogastric artery embolization in endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2001; 33:1193–1198.
11. Yano OJ, Morrissey N, Eisen L, Faries PL, Soundararajan K, Wan S, et al. Intentional internal iliac artery occlusion to facilitate endovascular repair of aortoiliac aneurysms. J Vasc Surg. 2001; 34:204–211.
12. Engelke C, Elford J, Morgan RA, Belli AM. Internal iliac artery embolization with bilateral occlusion before endovascular aortoiliac aneurysm repair-clinical outcome of simultaneous and sequential intervention. J Vasc Interv Radiol. 2002; 13:667–676.
13. Bratby MJ, Munneke GM, Belli AM, Loosemore TM, Loftus I, Thompson MM, et al. How safe is bilateral internal iliac artery embolization prior to EVAR. Cardiovasc Intervent Radiol. 2008; 31:246–253.
14. Rayt HS, Bown MJ, Lambert KV, Fishwick NG, McCarthy MJ, London NJ, et al. Buttock claudication and erectile dysfunction after internal iliac artery embolization in patients prior to endovascular aortic aneurysm repair. Cardiovasc Intervent Radiol. 2008; 31:728–734.
15. Lee WA, Berceli SA, Huber TS, Ozaki CK, Flynn TC, Seeger JM. Morbidity with retroperitoneal procedures during endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2003; 38:459–463.
16. McDonnell CO, Semmens JB, Allen YB, Jansen SJ, Brooks DM, Lawrence-Brown MM. Large iliac arteries: a high-risk group for endovascular aortic aneurysm repair. J Endovasc Ther. 2007; 14:625–629.
17. Torsello G, Schonefeld E, Osada N, Austermann M, Pennekamp C, Donas KP. Endovascular treatment of common iliac artery aneurysms using the bell-bottom technique: long-term results. J Endovasc Ther. 2010; 17:504–509.
18. Naughton PA, Park MS, Kheirelseid EA, O'Neill SM, Rodriguez HE, Morasch MD, et al. A comparative study of the bell-bottom technique vs hypogastric exclusion for the treatment of aneurysmal extension to the iliac bifurcation. J Vasc Surg. 2012; 55:956–962.
19. Karthikesalingam A, Hinchliffe RJ, Holt PJ, Boyle JR, Loftus IM, Thompson MM. Endovascular aneurysm repair with preservation of the internal iliac artery using the iliac branch graft device. Eur J Vasc Endovasc Surg. 2010; 39:285–294.
20. Lobato AC. Sandwich technique for aortoiliac aneurysms extending to the internal iliac artery or isolated common/internal iliac artery aneurysms: a new endovascular approach to preserve pelvic circulation. J Endovasc Ther. 2011; 18:106–111.
21. Lobato AC, Camacho-Lobato L. The sandwich technique to treat complex aortoiliac or isolated iliac aneurysms: results of midterm follow-up. J Vasc Surg. 2013; 57:2 Suppl. 26S–34S.
22. Abul-Khoudoud OR, Criado FJ, Wilson EP. Endovascular AAA repair: management strategies for the iliac artery. J Invasive Cardiol. 2000; 12:221–224.
23. Tillich M, Bell RE, Paik DS, Fleischmann D, Sofilos MC, Logan LJ, et al. Iliac arterial injuries after endovascular repair of abdominal aortic aneurysms: correlation with iliac curvature and diameter. Radiology. 2001; 219:129–136.
24. Carroccio A, Faries PL, Morrissey NJ, Teodorescu V, Burks JA, Gravereaux EC, et al. Predicting iliac limb occlusions after bifurcated aortic stent grafting: anatomic and device-related causes. J Vasc Surg. 2002; 36:679–684.
25. Fernandez JD, Craig JM, Garrett HE Jr, Burgar SR, Bush AJ. Endovascular management of iliac rupture during endovascular aneurysm repair. J Vasc Surg. 2009; 50:1293–1299.
26. Criado FJ. Iliac arterial conduits for endovascular access: technical considerations. J Endovasc Ther. 2007; 14:347–351.
27. Peterson BG, Matsumura JS. Internal endoconduit: an innovative technique to address unfavorable iliac artery anatomy encountered during thoracic endovascular aortic repair. J Vasc Surg. 2008; 47:441–445.
28. Jean-Baptiste E, Batt M, Azzaoui R, Koussa M, Hassen-Khodja R, Haulon S. A comparison of the mid-term results following the use of bifurcated and aorto-uni-iliac devices in the treatment of abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2009; 38:298–304.




 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download