Abstract
Purpose
The purpose of this study was to develop a porcine training model for laparoscopic choledochojejunostomy (CJ) that can act as a bridge between simulation models and actual surgery for novice surgeons. The feasibility of this model was evaluated.
Methods
Laparoscopic CJ using intracorporeal sutures was performed on ten animals by a surgical fellow with no experience in human laparoscopic CJ. A single layer of running sutures was placed in the posterior and anterior layers. Jejunojejunostomy was performed using a linear stapler, and the jejunal opening was closed using absorbable unidirectional sutures (V-Loc 180).
Results
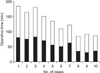
The average operation time was 131.3 ± 36.4 minutes, and the CJ time was 57.5 ± 18.4 minutes. Both the operation time and CJ time showed a steady decrease with an increasing number of cases. The average diameter of the CBD was 6.4 ± 0.8 mm. Of a total of ten animals, eight were sacrificed after the procedure. In two animals, a survival model was evaluated. Both pigs recovered completely and survived for two weeks, after which both animals were sacrificed. None of the animals exhibited any signs of bile leakage or anastomosis site stricture.
In the field of hepatobiliary surgery, choledochojejunostomy (CJ) is performed in cases where the common bile duct (CBD) is resected; CJ is performed for various reasons such as malignancy, several benign conditions such as choledochal cyst, or trauma. As in other fields of surgery, laparoscopic surgery is making rapid progress in hepatobiliary surgery. Many authors have reported on the feasibility of laparoscopic CJ [1,2]. However, hepatobiliary surgery has some inherent characteristics that complicate the transition to laparoscopic surgery, such as a narrow field of vision and risk of massive bleeding. Due to these factors, many hospitals still perform open surgery for procedures requiring CJ. Two additional major factors are the difficulty of intracorporeal suturing and anastomoses and the lack of an adequate practice method.
Indeed, for a novice surgeon to acquire advanced surgical techniques such as laparoscopic CJ, an adequate training model is needed. Surgical training programs utilize many different types of training models for this purpose, such as bench models, live animals, cadavers, or virtual reality simulators [3]. There have been studies evaluating the feasibility of bench models as well as studies evaluating cadaver models [4,5,6]. The usefulness of the live animal model has also been shown in many different fields [7,8]. Since the advent of robotic surgery, animal models have also been used to assess the feasibility of robotic surgery [9]. Compared to the bench model or the cadaver model, the live animal model has the advantage of excellent human tissue simulation.
Our purpose was to develop a live animal training model that can act as a bridge between bench simulation and actual surgery. Animal models that can be used have been reported previously, but this is the first training model using laparoscopic Roux-en-Y CJ as its main procedure. Here we introduce the results of our training model.
All operations were performed by a surgical fellow, with no experience in human laparoscopic CJ. All animals received care consistent with the guidelines of the Institutional Animal Care and Use Committee. The weight of animals was approximately 35 to 40 kg. Zoletil, ketamine, and xylazine were used for induction anesthesia. Zoletil was reconstituted with 2.5-mL ketamine (100 mg/mL) and 2.5-mL xylazine (100 mg/mL), and 0.03 ml/kg of this solution was administered intramuscularly. General anesthesia was maintained with inhalation anesthesia using 2.5% isoflurane.
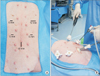
Trocar placement is shown in Fig. 1A. A 10-mm trocar was placed in the umbilicus, and CO2 gas was insufflated to create pneumoperitoneum. A 12-mm trocar and three 5-mm trocars were placed.
The procedure starts with the surgeon standing at the right side of the animal. A monofilament suture with a straight needle was passed through the liver, withdrawn from the abdomen, and tied extracorporeally to retract the right median lobe and left median lobe of the liver. The Treitz ligament was identified. The mesenteric vessels of the proximal jejunum designated as the CJ site were identified. Ultrasonic dissection was performed to divide the mesentery. A laparoscopic linear stapler was used to divide the jejunum.
The surgeon then moves to the left side of the animal. This position is ergonomically appropriate for performing laparoscopic CJ (Fig. 1B). Electrocautery and sharp dissection was used to dissect the hepatoduodenal ligament. The CBD was identified and divided with electrocautery. The distal CBD was closed with a 4-0 absorbable coated multifilament suture (Vicryl, Ethicon, Somerville, NJ, USA). Another 4-0 multifilament suture was used to anchor the Roux limb to the Glissonian sheath near the proximal CBD. Electrocautery was used to make an opening in the jejunum. This opening was widened with a dissector, based on the diameter of the CBD.
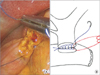
Approximately 15 cm of 4-0 absorbable coated monofilament suture (Monosyn, B. Braun, Melsungen, Germany) was introduced into the abdomen via the 10-mm trocar. Using this suture, the left edge of the bile duct and jejunal opening was sutured, with the knot outside of the lumen. Then, the jejunum was sutured from the outside to the inside, and the suture was placed inside the lumen. After this, the posterior layer was sutured in a running fashion until the right side edge of the bile duct and jejunal opening was reached. At the very edge, only the bile duct was sutured from the inside to the outside, and the suture was placed outside the lumen (Fig. 2). Another 15 cm of 4-0 monofilament suture was placed in the abdomen. The bile duct and jejunal opening were sutured in the same fashion immediately to the right of the posterior layer suture. With this suture, the anterior layer was sutured in the same running fashion until the right side edge was reached. At the very edge, the last suture was placed through both the bile duct and jejunum, leaving the suture outside the lumen. The posterior layer suture and anterior layer suture were tied. End to side single-layer CJ was completed.
Jejunojejunostomy was performed to complete the Roux-en-Y CJ. The proximal jejunum and the jejunum approximately 40-cm distal to the CJ were aligned. A Vicryl 4-0 suture was used to place a stay suture in both bowels. Electrocautery was used to create an opening in the two loops of jejunum near the stay suture. Through these openings, a linear stapler was inserted and fired, creating an anastomosis. The opening in the jejunum was closed using a 3-0 absorbable unidirectional suture (V-Loc 180, Covidien, Mansfield, MA, USA).
Laparoscopic CJ was performed in 10 animals. Fig. 3 shows the perioperative data. The average operation time was 131.3 ± 36.4 minutes, and the CJ time was 57.5 ± 18.4 minutes. The average diameter of the CBD was 6.4 ± 0.8 mm. Eight of the pigs were sacrificed upon completion of the procedure, and the anastomoses were examined for leaks. There were no grossly visible leakage sites in any animals. In two animals, a survival model was evaluated. Both animals recovered completely, and drank water within 24 hours. Both animals ingested standard pig chow within 48 hours. There were no postoperative complications or diet intolerance in either animal. On postoperative day 14, both animals were sacrificed, and the anastomosis sites were examined. None of the survival model animals exhibited any sign of bile leakage or anastomosis site stricture.
Roux-en-Y CJ is an important procedure in hepatobiliary and pancreatic surgery and is used in the treatment of various diseases. First, it is used when pancreaticoduodenectomy is performed in patients with borderline or low-grade malignancies. Second, it is used when palliative biliary bypass is performed in patients with unresectable periampullary cancer. Third, it is used as a biliary drainage procedure in cases of recurrent choledocholithiasis. Fourth, it is used in the treatment of choledochal cysts, after resection of the entire CBD. Lastly, Roux-en-Y CJ can be performed in cases with CBD injury.
The advent of laparoscopic Roux-en-Y CJ has been relatively recent, and in most cases, it is performed during open surgery. A long midline incision or subcostal incision is required for two reasons. First, the proximal CBD is in a fixed position at the liver hilum and is covered by the liver. Second, for a Rouxen-Y anastomosis, the jejunum below the transverse colon must be manipulated and anastomosed to the CBD, which has a relatively small caliber.
Other than these anatomical factors, a major additional factor that disturbs the transition from open surgery to laparoscopic surgery is the difficulty of intracorporeal suturing. In other types of gastrointestinal anastomoses, such as gastrojejunostomy or duodenojejunostomy, a stapling device can be used to avoid the process of intracorporeal suturing. However, due to the small caliber of the CBD and the anatomical characteristics of CJ, stapling is impossible, and intracorporeal suturing is essential for anastomosis.
Procedures that are based on advanced laparoscopic surgery such as laparoscopic CJ, including intracorporeal suturing, can be dangerous without adequate training. Accordingly, many types of training programs and simulation models have been developed. Bench models, cadaver models, and live animal models are types of training models. Various factors such as cost, ethical concerns, and human tissue simulation must be considered in choosing the right training model. When the bench model or the cadaver model is used, there is less ethical concern, but human tissue simulation can be suboptimal. In contrast, when live animal models are used, ethical concerns are raised, and specialized facilities are necessary [3]. However, in these models, human tissue simulation is excellent. Given these issues, it seems that the live animal models are adequate when training for advanced laparoscopic techniques such as laparoscopic CJ.
As noted above, some authors have described training models for laparoscopic CJ. As early as 1996, Schob et al. [10] described a porcine teaching model for laparoscopic CJ. The resected CBD was ligated, and a separate opening was created. Side to side CJ was performed using running sutures for the posterior layer and interrupted sutures for the anterior layer. Reed et al. [11] reported a porcine model of laparoscopic CJ and gastrojejunostomy, that can be an alternative to open palliative bypass in unresectable pancreatic cancer. In the training model described in this paper, the CBD was not resected, and a transverse choledochotomy was placed. Interrupted sutures were used to perform side to side CJ. In these two reports, the CJ procedures were both side to side anastomoses, and interrupted sutures were at least partially used. The usage of interrupted sutures may have prolonged the operative time, and may have added to the difficulty of the procedure. In 2008, Suzuki et al. [12] reported a porcine model for laparoscopic pancreaticoduodenectomy. The technique described is similar to the technique used in the present paper, in that a single layer end to side CJ was performed with running sutures. However, the main procedure of this paper was pancreaticoduodenectomy, and CJ was only a part of the reconstruction phase. In contrast, the training model introduced in this paper focuses on the laparoscopic CJ procedure.
Since the advent of robotic surgery, animal models using the robot have been developed [13,14]. Both papers described end to side CJ. One article described CJ using running sutures for both the posterior layer and the anterior layer [14], and the other described CJ using interrupted sutures for both layers [13]. The obvious distinction between these training models and the model introduced here is that robotic surgery was used. Despite the rapid progress of robotic surgery, many centers still do not perform robotic surgery. Additionally, in centers aiming for the transition to laparoscopic surgery, robotic surgery is a somewhat remote concept.
Although porcine anatomy is different from human anatomy in many aspects, there are no remarkable differences in performing laparoscopic Roux-en-Y CJ. The size of the pig provided a similar surgical environment, with comparable laparoscopic ergonomics. The location of the CBD was similar to that of the human CBD. As a result, dissection of the hepatoduodenal ligament was similar. In all animals, the CBD was of normal diameter, without any dilation. The procedure may have been slightly more difficult than in human cases because, in most human cases, the CBD is dilated. Although the porcine small intestine is longer than the human intestine, the division of the jejunum and the reconstruction phase were not difficult.
The authors have acquired several technical tips in performing laparoscopic CJ in the pig model. Using these tips, we were able to reduce the operative time. First, the use of anchor sutures to fix the jejunum to the Glissonian sheath near the proximal CBD aided in relieving anastomotic tension. This procedure also facilitates a more precise opening of the jejunum.
Second, in our experience, laparoscopic CJ can be comfortably performed with the operator standing to the left side of the patient. When performing open CJ, most operators stand to the right of the patient. However, because the CBD is located on the right side of the midline, it is ergonomically appropriate for the surgeon to stand on the left side of the patient while laparoscopically performing anastomoses.
Third, when choosing suture material, coated monofilament sutures seem to be better. Although multifilament sutures are easier to handle, monofilament sutures have more capacity to tighten the anastomosis after completion of sutures because of less friction. This characteristic can be beneficial for the patency of the anastomosis.
Among the 10 animals evaluated in this study, two animals were evaluated as a survival model. The two animals survived for 14 days and were then sacrificed. In the acute model, only the immediate results of a procedure can be evaluated. In contrast, in the survival model, the feasibility of the procedure can be assessed. This training model is a feasible procedure that can help train surgeons in acquiring the skills necessary for laparoscopic CJ.
In conclusion, the porcine training model introduced in this paper is an adequate model to practice laparoscopic CJ, and human tissue simulation is excellent.
Figures and Tables
References
1. Han HS, Yi NJ. Laparoscopic Roux-en-Y choledochojejunostomy for benign biliary disease. Surg Laparosc Endosc Percutan Tech. 2004; 14:80–84.
2. Gumbs AA, Hoffman JP. Laparoscopic radical cholecystectomy and Roux-en-Y choledochojejunostomy for gallbladder cancer. Surg Endosc. 2010; 24:1766–1768.
3. Reznick RK, MacRae H. Teaching surgical skills: changes in the wind. N Engl J Med. 2006; 355:2664–2669.
4. Hon SS, Ng SS, Lee JF, Li JC, Lo AW. In vitro porcine training model for colonic endoscopic submucosal dissection: an inexpensive and safe way to acquire a complex endoscopic technique. Surg Endosc. 2010; 24:2439–2443.
5. Narumi S, Toyoki Y, Ishido K, Kudo D, Umehara M, Kimura N, et al. Introduction of a simulation model for choledochoand pancreaticojejunostomy. Hepatogastroenterology. 2012; 59:2333–2334.
6. Bauer F, Rommel N, Kreutzer K, Weitz J, Wagenpfeil S, Gulati A, et al. A novel approach to teaching surgical skills to medical students using an ex vivo animal training model. J Surg Educ. 2014; 71:459–465.
7. Jeong SH, Park JH, Yoo MW, Choi SK, Hong SC, Jung EJ, et al. Feasibility of the transumbilical route compared with the transoral route in gastric upper body endoscopic submucosal dissection: a porcine model. Surg Endosc. 2014; 28:515–523.
8. Milsom JW, Trencheva K, Ezell P, Maggiori L, Pavoor R, Vitellaro M, et al. Feasibility and safety of laparoscopic colon surgery performed under intravenous sedation and local anesthesia using microinvasive (<3 mm) instruments: an acute and survival study on porcine model. Surg Innov. 2015; 22:131–136.
9. Jayaraman S, Quan D, Al-Ghamdi I, El-Deen F, Schlachta CM. Does robotic assistance improve efficiency in performing complex minimally invasive surgical procedures? Surg Endosc. 2010; 24:584–588.
10. Schob OM, Day PW, Josloff RK, Zucker KA. An experimental teaching model for laparoscopic choledochojejunostomy. Surg Laparosc Endosc. 1996; 6:341–347.
11. Reed DN Jr, Cacchione RN, Allen JW, Arlauskas V, Casey J, Larson GM, et al. Laparoscopic choledochojejunostomy and gastrojejunostomy in a porcine model. Surg Endosc. 2003; 17:86–88.
12. Suzuki O, Hirano S, Yano T, Okamura K, Hazama K, Shichinohe T, et al. Laparoscopic pancreaticoduodenectomy is effective in a porcine model. Surg Endosc. 2008; 22:2509–2513.
13. Laursen HB, Thorsoe HJ, Funch-Jensen P, Rokkjaer M, Yasuda Y, Mortensen FV. Robotic-assisted laparoscopic Roux-en-Y choledochojejunostomy in pigs. J Hepatobiliary Pancreat Surg. 2005; 12:167–172.
14. Ruurda JP, van Dongen KW, Dries J, Borel Rinkes IH, Broeders IA. Robot-assisted laparoscopic choledochojejunostomy. Surg Endosc. 2003; 17:1937–1942.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download