Abstract
Primary cutaneous apocrine carcinoma is a rare adnexal tumor of the skin that occurs mainly in the axilla, anogenital area while the scalp and the lower extremities, especially the thigh, are very unusual sites. However, clinical or pathologic characteristics have not been well established due to a paucity of this tumor. Herein, we report very unusual case of apocrine carcinoma present as a huge mass in the lateral thigh of 77-year-old woman, which was aggravated abruptly after an irritation by moxa treatment, with a brief review of the literature.
Primary cutaneous apocrine carcinoma is a rare adnexal tumor of the skin that occurs in the area with the greatest concentration of apocrine glands like the axillary region, scalp and groin [1]. As unusual occurring sites, nipples, finger tips and lips have been reported [2]. However, their clinical or pathologic characteristics have not been well established due to a paucity of this tumor.
Herein, we report a very unusual case of apocrine carcinoma occurring in the lateral thigh that was present as a huge subcutaneous mass with a brief review of the literature.
A 77-year-old female visited hospital due to a right thigh mass. The mass had been found as small nodule about 50 years ago with no specific treatment, but recently it grew rapidly after applying moxa treatment to the site. She had a medical history of diabetes mellitus, hyperthyroidism and angina and received bipolar hemiarthroplasy 4 years ago due to right femur neck fracture; the mass was distant from the previous operation site. On physical examination, there was a large, firm, nontender mass on the anterolateral side of thigh, measuring about 6 cm in the greatest dimension (Fig. 1A). The mass seemed to be movable over the deep lying tissue. The overlying skin showed hardening of surface, but any color change to suggest melanocytic or other skin cancer or abscess was not noted. Other mass-like lesions suspicious for metastasis were not found around the lesion, especially at the right inguinal area. Radiologically, enhanced CT on the thigh revealed an approximately 4.6 × 4.3-cm sized, oval shaped soft tissue lesion in the dermis and epidermis of the right thigh with no identifiable metastatic node at the inguinal area, showing slightly high attenuation on precontrast image with internal faint calcification and heterogeneous enhancement after contrast injection (Fig. 1B-D). Having an impression of soft tissue sarcoma or unknown metastatic tumors, a wide excision was done under the supine position with general anesthesia. The mass was completely excised with a gross minimum of 1 cm safety margin and primary skin closure was done with polypropylene 2-0. Hemovac was placed and removed at 3rd postoperative day. She was discharged at the sixth postoperative day without complication. She refused adjuvant treatment considering her old age and much comorbidity, but there was no sign of local recurrence and metastasis at 3 months, to date.
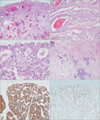
Microscopically, the mass was relatively well defined but focally infiltrative and the tumor cells arranged in a tubular, cribriform, nested pattern (Fig. 2A). Most lumens contain bloody and necrotic secretion (Fig. 2B). Pleomorphic tumor cells had abundant eosinophilic cytoplasm, round to oval nuclei, dispersed chromatin and inconspicuous nuclei; frequent mitoses were noted including atypical mitosis (Fig. 2C). Some fibrotic portions in the deeper portion of the mass showed infiltrative growth of tumor nest that resembled metastatic adenocarcinoma (Fig. 2D). There were multiple small calcifications that were noted radiologically, but necrosis was not found. Tumor cells were positive for CK7 and CK19, suggesting origin of skin appendage (Fig. 2E). Ki-67 proliferation index was estimated over 50% (Fig. 2F).
Primary cutaneous apocrine carcinoma is not common with any distinctive clinical features. It usually occurs as a single or multinodular mass, or plaque, and mainly in the axilla or anogenital region and rarely in the chest, nipple, finger or the scalp [1,3,4]. It can be sometimes confused with metastatic breast cancer or lymph node metastasis, especially if the mass was developed in an unusual site as in the present case. Metastasis to regional lymph nodes or distant metastasis has been reported in about 30% of the reported cases [1]. Although no formal clinical trial data are available, surgical excision with negative margin is considered as first choice at least for now; there is no consensus regarding the drugs or even the radiation dosages [2]. If the tumor was adequately treated and initial metastasis was not found, the prognosis might be good, based on the several reported cases [1,2,3].
For the diagnosis of primary cutaneous apocrine carcinoma, it depends histologically on apocrine differentiation of the tumor and clinically on a usual anatomic location where apocrine glands are numerous. A lower extremity is clinically an unusual site for developing apocrine carcinoma. Only a few cases presenting in the thigh have been reported [1,2,5]. Indeed, patients should be clinically evaluated for a history or clinical evidence of breast cancer because cutaneous apocrine carcinoma can be histologically indistinguishable from normal apocrine gland or apocrine carcinoma of female breast [6]. In the present case, the patient had no evidence or history of breast cancer.
Histologically, tumors should show decapitation secretion, the hallmark of apocrine glandular differentiation, an infiltrative margin and cytologic atypia, unacceptable for a benign neoplasm. Tumor cells with abundant granular, eosinophilic cytoplasm secret into luminal space.
On the review of cases of apocrine carcinoma by Robson et al. [1], classic apocrine can be diagnosed mainly based on its histologic features such as abundant granular, eosinophilic cytoplasm with luminal decapitation secretion, and immunohistochemical stains are not disease specific. Additionally, they suggested that the modified Bloom-Richardson grading of breast cancer also can be applied to primary cutaneous apocrine carcinoma.
Costa et al. [2] also reported a huge mass of apocrine carcinoma presented in the right thigh. He reviewed it in the therapeutic views and concluded that wide resection with free margins associated to regional lymphadenectomy is the first choice treatment.
Additionally, one considerable thing in our case is that the mass showed indolent course within about 50 years and grew abruptly after irritation by moxa treatment. Although there had been no available data that benign apocrine tumors developed into malignant tumors, we could consider the possibility that physical irritation might be associated with transformation to malignancy.
In conclusion, we report the first case of apocrine carcinoma in the right thigh presented as a huge mass in Korea. Furthermore, it will be worth noting that the tumor was aggravated after a moxa treatment, an uncommon treatment modality in western countries.
Figures and Tables
Fig. 1
(A) Grossly; large, firm, movable mass with thickened overlying skin is noted on anterolateral side of thigh, measuring about 6 cm in greatest dimension. (B) On precontrast CT, approximately 4.6 × 4.3-cm sized, oval shaped soft tissue mass is located in dermis and subcutis of right thigh having no connection with muscle. (C, D) After contrast injection, mass shows and heterogeneous enhancement and faint calcification.

Fig. 2
(A) At low power view, mass was relatively well defined but had focally infiltrative features. Tumor cell nests show decapitation secretion and glandular differentiation (H&E, ×12.5). (B) Some nests show tubular and cribriform arrangement with bloody luminal secretion (H&E, ×40). (C) Tumor cells have abundant eosinophilic cytoplasm, round to oval nuclei, dispersed chromatin and inconspicuous nuclei with frequent mitoses (H&E, ×400). (D) Deep portion of mass shows infiltrated tumor glands surround by sclerotic stroma (H&E, ×100). (E) Tumor cells are positive for CK19, suggesting origin of skin appendage (CK19, ×100). (F) High proliferation index is noted, estimating over 50% (Ki-67, ×100).
References
1. Robson A, Lazar AJ, Ben Nagi J, Hanby A, Grayson W, Feinmesser M, et al. Primary cutaneous apocrine carcinoma: a clinico-pathologic analysis of 24 cases. Am J Surg Pathol. 2008; 32:682–690.
2. Costa SR, Henriques AC, Horta SH. Apocrine gland carcinoma on the right thigh. Einstein (Sao Paulo). 2008; 6:478–480.
3. Katagiri Y, Ansai S. Two cases of cutaneous apocrine ductal carcinoma of the axilla. Case report and review of the literature. Dermatology. 1999; 199:332–337.
4. el-Domeiri AA, Brasfield RD, Huvos AG, Strong EW. Sweat gland carcinoma: a clinico-pathologic study of 83 patients. Ann Surg. 1971; 173:270–274.
5. Rutten A, Kutzner H, Mentzel T, Hantschke M, Eckert F, Angulo J, et al. Primary cutaneous cribriform apocrine carcinoma: a clinicopathologic and immunohistochemical study of 26 cases of an under-recognized cutaneous adnexal neoplasm. J Am Acad Dermatol. 2009; 61:644–651.
6. Hur H, Ahn YJ, Park SH, Kim JH, Koo JS, Park BW. Clinicopathologic characteristics of apocrine breast carcinoma. J Korean Surg Soc. 2009; 77:43–49.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download